Consider the structure of formaldehyde: a) Identify the type of bonds that form the C = O
Question:
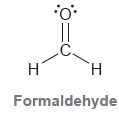
a) Identify the type of bonds that form the C = O double bond.
b) Identify the atomic orbitals that form each C = H bond.
c) What type of atomic orbitals do the lone pairs occupy?
Transcribed Image Text:
.C. H. Formaldehyde
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a The CO bond of formaldehyde is comprised of one sigma b...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the structure of the catnip ingredient nepetalactone (page 305). a. Show with dotted lines that the structure is composed of two isoprene units. b. Circle the stereogenic centers and...
-
Consider the structure of prostaglandin E2 shown on page 451. a. How many stereogenic centers are present? b. What is the configuration (R or S) of each? c. What is the configuration of the double...
-
Consider the structure of the following compound: (a) When this compound is treated with bromine under conditions that favor monobromination, two stereoisomeric products are obtained. Draw them, and...
-
The input file for this assignment is Weekly_Gas_Average.txt. The file contains the average gas price for each week of the year. Write a program that reads the gas prices from the file into an...
-
How is a bank a manufacturing business? (Think of a bank's "production function.")
-
Which answer best describes the direction, form, and strength of the association between the distance of a hike (in miles) and the time it takes to complete the hike (in minutes). A. Positive,...
-
Event A: Randomly select a person between 18 and 24 years old. Event B: Randomly select a person who drives a convertible.
-
Finman Company designated Jill Holland as petty cash custodian and established a petty cash fund of $200. The fund is reimbursed when the cash in the fund is at $15. Petty cash receipts indicate...
-
Allowance Method of Accounting for Bad DebtsComparison of the Two Approaches Kandel Company had the following data available for 2017 (before making any adjustments): Accounts receivable, 12/31/17...
-
Researchers interested in the effects of caffeine on anxiety have randomly assigned participants to one of two conditions in a studythe nocaffeine condition or the caffeine condition. After drinking...
-
In each of the following transformations, identify whether the starting material has been oxidized, reduced, or neither. Try to determine the answer without calculating Oxidation states, and then use...
-
Identify which two compounds below are constitutional isomers: (CH].C,H, (CH),c, (CH),,
-
List the project quality tools you expect to use on your project. Tell where you plan to use each tool and why it is important.
-
Use the information below to answer the next question. Below are different graphs that could represent the magnitude of an Electric Field from a source. Teza E Distance E 4 Tza E Taza 2 Distance 5 3...
-
Factor out the GCF: 36c5 +54c8
-
Demonstrate that a circle with a radius of r has a circumference of 2 pi ( r ) . HINT: Begin by examining the equation for the upper semicircle, utilize the arc length formula, and then double the...
-
Graph the function f(x) = 3.x - 7.
-
Vine plc. produces a single product. The following information on inventory, purchases, and sales are available for the month of January 2018. DATE TRANSACTION NUMBER OF UNITS UNIT COST...
-
Find the domain of f and write it in set- builder or interval notation. f(x) = ln(2x - 4)
-
10m solution. If Ka(HA) = 10 then pOH of solution will be [Given : log4=0.6] (A) 6.7 (B) Greater than 6.7 & less than 7.0 (C) Greater 7.0 & less than 7.3 (D) Greater than 7.3
-
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethyl magnesium bromide. Is the product chiral? Is it optically active? Explain.
-
Give IUPAC names for the following compounds: (a) (b) (c) CH CHCHCH2CH CH2CH2CH3 H-CH2CHCH20H (d) (e) Ph. (f) Br NEC-
-
Draw and name the eight isomeric alcohols with formula C5H12O.
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


