In each of the following transformations, identify whether the starting material has been oxidized, reduced, or neither.
Question:
In each of the following transformations, identify whether the starting material has been oxidized, reduced, or neither. Try to determine the answer without calculating Oxidation states, and then use the calculations to see if your intuition was correct.
a.
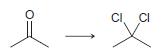
b.
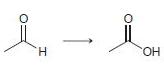
c.
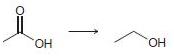
d.
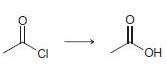
e.
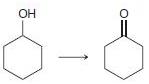
f.
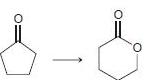
CI. CI - 3
Step by Step Answer:
a 2 2 the starting material is neither oxidized nor redu...View the full answer



Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Sciences questions
-
Determine whether refrigerant R-22 in each of the following states is a compressed liquid, a superheated vapor, or a mixture of saturated liquid and vapor.
-
Calculating Break-Even in each of the following cases, calculate the accounting break-even and the cash break-even points. Ignore any tax effects in calculating the cash break-even. Unit Price Unit...
-
Determine whether the taxpayer in each of the following situations has a claim of right to the income received: a. Sulley's Spa Spot sells hot tubs that have a 2-year warranty. The warranty provides...
-
Write a function my_ieee_2_dec(ieee), where icce is a string contains 64 char- acters of ones and zeros representing a 64-bit IEEE754 number. The output should be d, the equivalent decimal...
-
Describe a repurchase agreement between a bank and one of its corporate depositors. How do both stand to gain?
-
Are college students more likely to be on their cell phone at certain times of day than other times as they walk across their campus? Student researchers explored this question. Of the 221 students...
-
Event A: Randomly select a student with a birthday in April. Event B: Randomly select a student with a birthday in May.
-
TeleTech Corporation manufactures two different fax machines for the business market. Cost estimates for the two models for the current year are as follows: The firms management expects to operate at...
-
The following information relates to Hogs Back Falls Ltd's inventory transactions during the month of February Units Cost/Unit Amount Feb. 1 Beginning inventory 6,800 $21.00 $142,800 10 Sale 5.900 14...
-
A solid plastic sphere falls towards the Earth. The diagram below shows the speed-time graph of the fall up to the point where the sphere hits the Earth's surface. a. Describe in detail the motion of...
-
When butyl bromide is treated with sodium iodide in ethanol, the concentration of iodide quickly decreases but then slowly returns to its original concentration. Identify the major product of the...
-
Consider the structure of formaldehyde: a) Identify the type of bonds that form the C = O double bond. b) Identify the atomic orbitals that form each C = H bond. c) What type of atomic orbitals do...
-
What mass of phosphorus is present in 5.00 moles of each of the compounds in Exercise 54? Data in Exercise 54? Calculate the molar mass of the following substances. a. b. Ca 3 (PO 4 ) 2 c. Na 2 HPO 4...
-
Use the Comprehensive Annual Financial Report for the Village of Arlington Heights (please look up this content) for the year ended December 31, 2018, to answer questions 8-20. All questions are on...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Two wires lie perpendicular to the plane of the screen and carry equal magnitudes of electric current in the directions shown. Point P is equidistant from the two wires. The distance between each of...
-
Your firm has recently been appointed as auditors of Kentronics Ltd , a large company which markets sophisticated electronic equipment for heavy industry as well as the mining equipment industry. The...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Expand the expression. If possible, write your answer without exponents. In 3k
-
General Electric Capital, a division of General Electric, uses long-term debt extensively. In a recent year, GE Capital issued $11 billion in long-term debt to investors, then within days filed legal...
-
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (i) Na...
-
Predict the product from reaction of the following substance (reddish brown = Br) with: (a) PBr3 (b) Aqueous H2SO4 (c) SOCl2 (d) PCC (e) Br2,FeBr3
-
Predict the product from reaction of the following substance with: (a) NaBH4 then H3O+ (b) LiAlH4 then H3O+ (c) CH3CH2 MgBr; then H3O+
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


