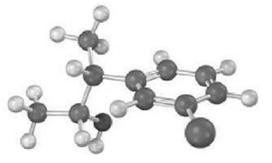
Predict the product from reaction of the following substance (reddish brown = Br) with: (a) PBr3 (b)
Question:
Predict the product from reaction of the following substance (reddish brown = Br) with:
(a) PBr3
(b) Aqueous H2SO4
(c) SOCl2
(d) PCC
(e) Br2,FeBr3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
a b HC c H3C H CH3 H3C H OH H CH3 CH3 aqueous HSO4 ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the product from reaction of the following substance with: (a) NaBH4 then H3O+ (b) LiAlH4 then H3O+ (c) CH3CH2 MgBr; then H3O+
-
Predict the product from the SN2 reaction of a. Trans-4-methylcyclohexyl bromide with cyanide ion. b. (S)-2-bromopentane with cyanide ion. c. (R)-2-chlorobutane with NaSH.
-
Predict the product from the reaction of phenylmagnesium bromide (C6H5MgBr) with benzoyl chloride (C6H5COCl).
-
In July 2009, Malcolm bought a piece of land for 40,000. In June 2016 he sold part of the land for 11,000. This disposal was not caused by a compulsory purchase and was his only disposal of land in...
-
Do you think it is better to concentrate on one source of competitive advantage (cost versus differentiation versus speed) or to nurture all three in a firm's operation?
-
1. Using the 4As framework in Figure 12.1, analyze the considerations that went into the development of the Gillette Guard razor for the Indian market? Figure 12.1 THE 4 As: The 4 As of addressing...
-
10. Provide computations to explain the $305,800 Investment in Son account balance on December 31, 2017.
-
Krause Company on January 1, 2012, enters into a five-year noncancelable lease, with four renewal options of one year each, for equipment having an estimated useful life of 10 years and a fair value...
-
Cheyenne Corporation issued $600,000, 7%, 20-year bonds on January 1, 2022, for $541,091. This price resulted in an effective-interest rate of 8% on the bonds. Interest is payable annually on January...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (i) Na...
-
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethyl magnesium bromide. Is the product chiral? Is it optically active? Explain.
-
A 1500-kg sedan goes through a wide intersection traveling from north to south when it is hit by a 2200-kg SUV traveling from east to west. The two cars become enmeshed due to the impact and slide as...
-
Case study: Sun City - improving operations performance to enhance guest experience 1. Describe how Sun City implements the five operations performance objectives or principles. 2. Using your...
-
What recommendations do you have to increase the likelihood of success? E.g., how would you reduce the likelihood of having to go back to A4? How would you reduce the impact of having to go back to...
-
Problem 4 An electrically heated, square plate (0.4mx 0.4 mx0.005 m) is suspended in air of temperature Too = 20C. Find the electrical power needed to maintain the plate at T=95C if the plate is (a)...
-
Number of units Unit Cost Sales Beginning inventory 800 $50 Purchased 600 $52 Sold 400 $80 Sold 350 $90 Ending inventory 650 In the table below, calculate the dollar value for the period for each of...
-
10. Dr. D went to MGM Springfield casino while the class was taking their midterm exam. He played a Konami machine entitled 88 Fortunes. A slot attendant accidently left the slot manual next to the...
-
How will Netflixs functional strategies have to support its competitive strategy? Explain.
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
Arrange the following substances in the expected order of increasing melting point: KI, Ne, K 2 SO 4 , C 3 H 8 , CH 3 CH 2 OH,MgO, CH 2 OHCHOHCH 2 OH.
-
Tell what is meant by a homologous series and illustrate your answer by writing structures for a homologous series of alkyl halides.
-
Four different cycloalkenes will all yield methylcyclopentane when subjected to catalytic hydrogenation. What are their structures? Show the reactions.
-
(a) Three different alkenes yield 2-methylbutane when they are hydrogenated in the presence of a metal catalyst. Give their structural formulas and write equations for the reactions involved. (b) One...
-
Discuss how debt restructuring, settlement, or modification works. Discuss the journal entries for debtor and creditor
-
Could CNL be a viable business? If so, under what conditions and what level of production (and, since production is directly related to production workers, employees)? All information provided for...
-
the internal operation rulea of cooperation are known As ?

Study smarter with the SolutionInn App


