Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been
Question:
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (i) Na metal, (ii) SOCI2, and (iii) pyridiniumchlorochromate.
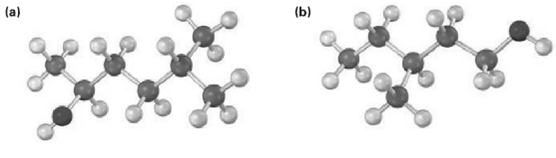
Transcribed Image Text:
(ь) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
The reduction product is a racemic mixture a ...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw the structure of each compound. (a) o-nitroanisole (b) 2, 4-dimethoxyphenol (c) p-aminobenzoic acid (d) 4-nitroaniline (e) m-chlorotoluene (f) p-divinylbenzene (g) p-bromostyrene (h) 3,...
-
Draw the structure of each possible dichloride that can be used to prepare the following alkyne via elimination:
-
Draw the structure of the following compounds: a. 2-propyloxirane b. Cyclohexene oxide c. 2,2,3,3-tetramethyloxirane d. 2,3-epoxy-2-methylpentane
-
In 2020-21, a taxpayer makes a number of disposals, as listed below. Which of these disposals would be exempt from CGT? (a) An antique table sold for 5,000. (b) A watercolour painting sold at...
-
How does market focus help a business create competitive advantage? What risks accompany such a posture?
-
Consider a monopolist who sells a product that contains two attributes A and B. Each of these attributes can be of high or of low quality. Low quality gives utility u i = 0 and high quality utility u...
-
9. What was the amount of patents at December 31, 2016? Show computations.
-
The following is the post-closing trial balance for the Whitlow Manufacturing Corporation as of December 31, 2010. The following transactions occurred during January 2011: Jan.1 Sold merchandise for...
-
The following table contains descriptions of errors related to inventory accounted for under a periodic inventory recordkeeping system and for property plant & equipment You need to show your work to...
-
A monopole antenna extends vertically over a perfectly conducting plane, and has a linear current distribution. If the length of the antenna is 0.01, what value of I 0 is required to (a) Provide a...
-
Give IUPAC names for the following compounds: (a) (b) (c) (d)
-
Predict the product from reaction of the following substance (reddish brown = Br) with: (a) PBr3 (b) Aqueous H2SO4 (c) SOCl2 (d) PCC (e) Br2,FeBr3
-
In Fig. 11.7e note that the pressure is not uniform across the diameter of the compact. Explain the reasons for this variation?
-
Complete the exercises on the following website. Remember to type your answers in word or excel, screenshot, or phone pic as your work. The site does not save your answers. Upload your work on...
-
There are many management theories that are utilized in organizations. These theories were developed by scholars in the management discipline. One individual was responsible for identifying the major...
-
An increase in the price and a decrease of the quantity of Paclitaxel (an anti-cancer drug) could be caused by which of the following? Select one: O a. an increase in the number of people being...
-
At December 31, 2023, Cord Company's plant asset and accumulated depreciation and amortization accounts had balances as follows: Category Land Land improvements Buildings Equipment Automobiles and...
-
Assume that the following table represents the sales figures for the five largest firms in the industry. Compute the HHI for the industry (assuming the industry contains just these five firms). Sales...
-
What competitive advantage(s) do you think Netflix has? Have its resources, capabilities, or core competencies contributed to its competitive advantage(s)? Explain.
-
Comptech Ltd is a manufacturer of optical equipment. In September 2019, Ed Thompson the Chief Research Officer, attended a conference in Switzerland that focused on optical developments for the 21st...
-
If the triple point pressure of a substance is greater than 1 atm, which two of the following conclusions are valid? (a) The solid and liquid states of the substance cannot coexist at equilibrium....
-
Write structures for the following bicyclic alkanes: (a) Bicyclo [1.1.0] butane (b) Bicyclo [2.1.0] pentane (c) 2-Chlorobicyclo [3.2.0] heptane (d) 7-Methylbicyclo [2.2.1] heptane
-
Draw bond-line formulas and give IUPAC substitutive names for all of the isomeric alcohols with the formulas (a) C4H10O and (b) C5H12O.
-
A spiro ring junction is one where two rings that share no bonds originate from a single carbon atom. Alkanes containing such a ring junction are called spiranes. (a) For the case of bicyclic...
-
4 Exercise 9-6 (Algo) Lower of cost or market [LO9-1) 75 Tatum Company has four products in its inventory. Information about the December 31, 2021, Inventory is as follows: oints Product Total Cost...
-
A real estate investment is expected to return to its owner $3,500 per year for 16 years after expenses. At the end of year 16, the property is expected to be sold for $49,000. Assuming the required...
-
You borrowed $15,000 for buying a new car from a bank at an interest rate of 12% compounded monthly. This loan will be repaid in 48 equal monthly installments over four years. Immediately after the...

Study smarter with the SolutionInn App


