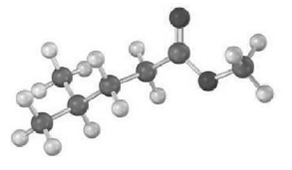
Predict the product from reaction of the following substance with: (a) NaBH4 then H3O+ (b) LiAlH4 then
Question:
Predict the product from reaction of the following substance with:
(a) NaBH4 then H3O+
(b) LiAlH4 then H3O+
(c) CH3CH2 MgBr; then H3O+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
a b c CH3 i CH3CHCHCHCOCH 3 CH3 CH3C...View the full answer

Answered By

RADHIKA MEENAKAR
I am a qualified indian Company Secretary along with Masters in finance with over 6 plus years of professional experience. Apart from this i am a certified accounts and finance tutor on many online platforms.
My Linkedin profile link is here https://www.linkedin.com/in/radhika-meenakar-88b9808a/
5.00+
12+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the product from reaction of the following substance (reddish brown = Br) with: (a) PBr3 (b) Aqueous H2SO4 (c) SOCl2 (d) PCC (e) Br2,FeBr3
-
Predict the product from the SN2 reaction of a. Trans-4-methylcyclohexyl bromide with cyanide ion. b. (S)-2-bromopentane with cyanide ion. c. (R)-2-chlorobutane with NaSH.
-
Predict the product from the reaction of phenylmagnesium bromide (C6H5MgBr) with benzoyl chloride (C6H5COCl).
-
Melissa is a sole trader. Her capital gains and capital losses for 2020-21 are 27,000 and 700 respectively. She has capital losses brought forward from 2019-20 of 12,900 and she also has unrelieved...
-
What are three ways a firm can incorporate the advantage of speed in its business? Use Exhibit 8.6 to help you answer this question. Can you give an example of a company that has done this?
-
What is the difference between recycling and lateral cycling?
-
PR 6-1 How should a company treat intercompany sales of assets in the consolidated financial statements?
-
Consider the following transactional data for the first month of operations for Shine King Cleaning. Nov. 1 Stockholders contributed $ 35,000 and a truck, with a market value of $ 8,000, to the...
-
A 30-year maturity, 8% coupon bond paying coupons semiannually is callable in five years at a call premium of 9%. The bond currently sells at a yield to maturity of 5%. What is the yield to call?
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (i) Na...
-
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethyl magnesium bromide. Is the product chiral? Is it optically active? Explain.
-
Design of Management Control System; Review of Chapters 22 and 23 Western Pants, Inc., is one of Americas oldest clothing firms. Founded in the midnineteenth century, the firm weathered lean years...
-
10.) Steam enters a well-insulated turbine at 6 MPa, 400C and expands to 200 kPa, saturated vapor at a rate of 10 kg/s. (a) Draw a schematic of the process (5 pts). (b) Determine the exergy...
-
4. [8 marks] The tides in the Bay of Fundy are some of the largest in the world. The height, h(t), of the tide in meters after t hourse can be modeled by 39 h(t) = 25 con (77) + 30 4 COS 6 (a) What...
-
Wolfe, Inc. had credit sales for the period of $144,000. The balance in Allowance for Doubtful Accounts is a debit of $653. If Wolfe estimates that 2% of credit sales will be uncollectible, what is...
-
Water at 20C is to be pumped from a reservoir (ZA = 5 m) to another reservoir at a higher elevation (ZB = 13 m) through two 36-m- long pipes connected in parallel as shown. The pipes are made of...
-
Delph Company uses a job-order costing system with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 53,000 machine-hours would...
-
What do you think Netflix is going to have to do to maintain its competitive position, especially as its industry changes?
-
What mass of KBr (in grams) should you use to make 350.0 mL of a 1.30 M KBr solution?
-
The normal boiling point of acetone is 56.2 C, and the molar heat of vaporization is 32.0 kJ mol 1 . What is the boiling temperature of acetone under a pressure of 50.0 mmHg?
-
An alkane with the formula C6H14 can be prepared by hydrogenation of either of only two precursor alkenes having the formula C6H12. Write the structure of this alkane, give its IUPAC name, and show...
-
Rank the following compounds in order of increasing stability based on relative ring strain.
-
Write the structures of two chair conformations of 1-tert-butyl-1-methylcyclohexane. Which conformation is more stable? Explain your answer.
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)

Study smarter with the SolutionInn App


