What mass of phosphorus is present in 5.00 moles of each of the compounds in Exercise 54?
Question:
What mass of phosphorus is present in 5.00 moles of each of the compounds in Exercise 54?
Data in Exercise 54?
Calculate the molar mass of the following substances.
a. 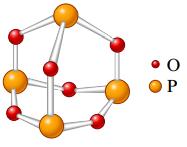
b. Ca3(PO4)2
c. Na2HPO4
Transcribed Image Text:
0 эр
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
Molecular compound PO 1 mole PO 36460 gmol x 500 moles 17...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
What mass of phosphorus is needed to dope l.0 g of silicon to the extent described in Sample Problem 41-6?
-
Phosphorus is present in seawater to the extent of 0.07 ppm by mass. If the phosphorus is present as phosphate, PO43-calculate the corresponding molar concentration of phosphate in seawater.
-
What are the moles of substances present at equilibrium at 450oC if 1.00 mol N2 and 4.00 mol H2 in a 10.0-L vessel react according to the following equation? The equilibrium constant Kc is 0.153 at...
-
If the working papers correlating with this textbook are not used, omit Problem 2-5B. The following records of A-Aall Electronic Repair are presented in the working papers: Journal containing...
-
Choose an appropriate forecasting technique for the data in the Excel file Federal Funds Rates and find the best forecasting model. Explain how you would use the model to forecast and how far into...
-
Determine if the sets of vectors in the given exercise are linearly independent by converting the vectors to row vectors and using the method of Example 2.25 and Theorem 2.7. For any sets that are...
-
Prepare journal entries to record the following transactions and events of Kodan Company. 2008 Jan. 2 Purchased 30,000 shares of Goreten Co. common stock for $408,000 cash plus a brokers fee of...
-
What type of intermediary is Li & Fung for its customers? Is it an agent, a wholesaler, a manufacturers agent, jobber, or some other choice? What evidence can you offer to support your choice?
-
XYZ Company makes one product and has calculated the following amounts for direct labor: AH x AR = $84,000; AH x SR = $83,000; SH x SR = $85,000. Compute the total labor cost variance.
-
Like many married couples, Morgan and Thomas Jensen are trying their best to save for two important investment objectives: (1) an education fund to put their two children through college; and (2) a...
-
Using the general solubility rules given in Table 4.1, name three reagents that would form precipitates with each of the following ions in aqueous solution. Write the net ionic equation for each of...
-
What mass of compound is present in 5.00 moles of each of the compounds in Exercise 54? Data In Exercise 54? Calculate the molar mass of the following substances. a. b. Ca 3 (PO 4 ) 2 c. Na 2 HPO 4 0
-
What role does the accountant play in the decision-making process?
-
Arizona Corp. had the following account balances at 12/1/19: Receivables: $96,000; Inventory: $240,000; Land: $720,000; Building: $600,000; Liabilities: $480,000; Common stock: $120,000; Additional...
-
Construct a 90% confidence interval for the population standard deviation o at Bank A. Bank A 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Margin of Error For the poll described in Exercise 1, describe what is meant by the statement that "the margin of error was given as +3.5 percentage points."
-
1) Explain what the critical issue was in the Uber decisions in the First Circuit (Culliane case), and the Second Circuit (Myer case), and how each court, looking at the same facts, came to opposite...
-
IFRS LEASE 1. Kappa Berhad enters into a 10-year lease on 1 January 2020. Kappa Berhad incurred the following costs in respect of the lease: RM2,500 legal fees RM15,000 deposit made at the...
-
A production supervisor at a company that makes eco-friendly cleaning supplies learns about the factors that should influence the span of control. The supervisor believes that his own span of control...
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Show the products of thesereactions: CH3 HBr a) CH,CHCH-CH2 HBr b) PhC CH 2 HBr
-
Show the products of thesereactions: H,SO,, H,SO4 b) + H,0 + H,0 H,SO. + HO
-
Show all of these steps in the mechanism for the addition of water to propene catalyzed by sulfuric acid. Explain whether propene or phenylethene (PhCH = CH2) has a faster rate in this reaction:
-
Estimate the intrinsic value of the stock company ABC. Dividends were just paid at $8 per share and are expected to grow by 5%. You require 20% on this stock given its volatile characteristics. If...
-
Crane, Inc., a resort management company, is refurbishing one of its hotels at a cost of $6,794,207. Management expects that this will lead to additional cash flows of $1,560,000 for the next six...
-
Match each of the following transactions with the applicable internal control principle that is being violated

Study smarter with the SolutionInn App


