Consider the structure of N,N-dimethylformamide (DMF): We might expect the two methyl groups to be equivalent; however,
Question:
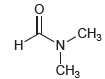
We might expect the two methyl groups to be equivalent; however, both the proton and carbon NMR spectra of DMF show two separate signals for the methyl groups. Propose an explanation for the nonequivalence of the methyl groups. Would you expect the signals to collapse into one signal at high temperature?
Transcribed Image Text:
CHз N. Н CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
NNdimethylformamide DMF has several resonance structures Consider the thi...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the structure of the catnip ingredient nepetalactone (page 305). a. Show with dotted lines that the structure is composed of two isoprene units. b. Circle the stereogenic centers and...
-
Consider the structure of prostaglandin E2 shown on page 451. a. How many stereogenic centers are present? b. What is the configuration (R or S) of each? c. What is the configuration of the double...
-
Consider the structure of the following compound: (a) When this compound is treated with bromine under conditions that favor monobromination, two stereoisomeric products are obtained. Draw them, and...
-
develop a trial balance, complete with an appropriate three-line heading 1. Bought office equipment from Peckoff Equipment Co. on 30-day credit terms, $1,390. Purchase Invoice #2071. Ledger Entries:...
-
What are three examples of code of ethics outlined by the Association for Computing Machinery (ACM)?
-
For the International Chef, Inc. scenario in Problem 13.27, obtain the Solver Sensitivity Report and write a short memo to the president, Kathy Chung, explaining the sensitivity information in...
-
Describe how change can be viewed as a process and the emotional responses people might have when faced with change. AppendixLO1
-
Mini-Case Study: The Back to School Crunch at Global Green Books Publishing Global Green Books Publishing is a successful printing and publishing company. Just two years old, it has taken on a great...
-
Each of the following scenarios involves a possible violation(s) of the Code of Professional Conduct (CPC). Discuss/explain any violations in the context of the CPC. (You do not need to cite the...
-
University endowments are financial assets that are donated by supporters to be used to provide income to universities. There is a large discrepancy in the size of university endowments. The...
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
Consider the structure of phenol: The chemical shift of the hydroxyl proton is found to be sensitive to the concentration of phenol. In a concentrated solution, the hydroxyl proton produces a signal...
-
19.8 Some have suggested that managers should negotiate transfer prices. What are the disadvantages of a negotiated transfer-pricing system? ;
-
Obtain the phase trajectories for a system governed by the equation \[\ddot{x}+0.4 \dot{x}+0.8 x=0\] with the initial conditions \(x(0)=2\) and \(\dot{x}(0)=1\) using the method of isoclines.
-
Indicate whether each of the following accounts normally has a debit balance or a credit balance. a. Land b. Dividends c. Accounts Payable d. Unearned Revenue e. Consulting Revenue f. Salaries...
-
Indicate whether each of the following accounts normally has a debit or credit balance. a. Common Stock b. Retained Earnings c. Land d. Accounts Receivable e. Insurance Expense f. Cash g. Dividends...
-
Match each of the items in the left column with the LO5, 6 appropriate annual report component from the right column: 1. The company's total liabilities 2. The sources of cash during the period 3. An...
-
Allegra Company has sales of \($167,000\) and a bicak-even sales point of \($123,000\). Compute Allegra s margin of safety and its margin of safety ratio.
-
Determine mentally an integer n so that the logarithm is between n and n + 1. Check your result with a calculator. (a) log 79 (c) log 5 (b) log 500 (d) log 0.5
-
1. Which of the four major types of information systems do you think is the most valuable to an organization? 2. How do you critically associate the ideas of business agility and business efficiency...
-
Rank the following sets of substituents in order of priority according to the Cahn-lngold?Prelog sequence rules: (a) -CH, -Br, -,-1 (b) -, -, -, -2 (c) -, -2CH3, -, - (d) -CH, -H2H, -H2H2, -H (e)...
-
An Assign E or Z configuration to each of the following alkenes: ( - C (a) H2 C=C C=C H3C ci OCH3 (c) (d) CH3O2C CH=CH2 NC CH C=C CH3CH2 CH2 HO2C CH-CH
-
Name the following cycloalkenes: (c) (b) (a) CH (f) (e) (d)
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


