Consider the structure of the compound below. (a) Identify each position where an S N 2 reaction
Question:
(a) Identify each position where an SN2 reaction is likely to occur.
(b) Identify each position where an SN1 reaction is likely to occur.
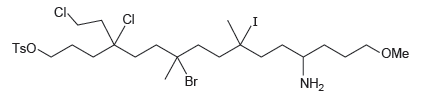
Transcribed Image Text:
Cl CI TsO. OMe Br NH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
a b ...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the structure of the following compound: (a) When this compound is treated with bromine under conditions that favor monobromination, two stereoisomeric products are obtained. Draw them, and...
-
Consider the structure of the azo dye called alizarine yellow R (below). Show the reagents you would use to prepare this compound via an azo coupling process. .N. N' O2N
-
Consider the structure of formaldehyde: a) Identify the type of bonds that form the C = O double bond. b) Identify the atomic orbitals that form each C = H bond. c) What type of atomic orbitals do...
-
In Exercises 1126, determine whether each equation defines y as a function of x. x + y = 16
-
Here is the topic "Capital Budgeting and Risk Analysis" respond to the following questions: * From the e-Activity, analyze the reasons why the short-term project that you have chosen might be ranked...
-
HR and hiring managers often find themselves swamped by rsums because they are so easy to send with a click of a button. Some large retailers can get a million or more rsums a year. Even small...
-
In assessing the relationship between two companies, how is control determined?
-
Cost-Cutting Proposals Chatman Machine Shop is considering a four-year project to improve its production efficiency. Buying a new machine press for $530,000 is estimated to result in $205,000 in...
-
Barlow Company manufactures three products: A, B and C The selling price variable costs and contribution margin for one unit of each product follow Product $215.00 $152.00 $210.00 Selling price Less:...
-
Refer to the adjusted trial balance of Green Lawns, Inc., illustrated in Exercise 5.2 to respond to the following items. a. Prepare all necessary closing entries at December 31, current year. b....
-
Identify the number of steps (patterns) for the mechanisms in Problems. For example, the patterns for the first two are: a: ±H ± LG Nuc attack This mechanism exhibits a proton transfer...
-
Does each of the following solvents favor an SN2 reaction or an SN1 reaction? a) b) c) d) (e) MeOH (f ) CH 3 CN (g) HMPA (h) NH 3 %24
-
Prepare journal entries for transactions of a process manufacturer. AppendixLO1
-
What is the difference between corporate and clinical? How do they differ? Can they both have the same outcome? Include a reference list that supports your stance of no fewer than 3 scholarly...
-
How do we attain the desire for the freedom to purse one's passions, the desire for economic security and well-being, the desire for hope and progress in one's life utilizing higher-order thinking
-
Instructions FNCE 625 - Investment Analysis and Management Group Project - Case Study Guideline Introduction: In this group assignment, each team will collaboratively make a comprehensive report and...
-
21) The EOQ model is solved using calculus but the key intuition is that relevant total costs are minimized when relevant ordering costs equal relevant carrying costs. 22) Safety stock is used as a...
-
In the long-term, what do you recommend as overall policy in order to reduce or avoid the kinds of PPE shortages that occurred during the different waves of the COVID virus? In simple terms, how...
-
Write 3 + 4i / 1 - i in standard form.
-
Write a paper about medication error system 2016.
-
Although monosaccharides undergo complex isomerizations in base (Section 22.5A), aldonic acids are epimerized specifically at C2 when they are heated with pyridine. Show how you could make use of...
-
Draw the β-pyranose form of (a) in its lowest energy chair conformation, and a Fischer projection for (b). (a) (b) CHO CH20H HO OH OH OH Ho Ho OH
-
The most stable conformation of most aldopyranoses is one in which the largest group, the iCH2OH group, is equatorial. However, d-idopyranose exists primarily in a conformation with an axial --CH2OH...
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


