Consider the structures of the following alkyl chlorides: a. Which compound would you expect to undergo an
Question:
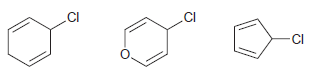
a. Which compound would you expect to undergo an SN1 process most readily? Justify your choice.
b Which compound would you expect to undergo an SN1 process least readily? Justify your choice.
Transcribed Image Text:
.CI .CI CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
a Loss of the leaving gr...View the full answer

Answered By

Subash Murugaih
I am leading expert in this web site couple of years and My clients are much happy with my works and services.
4.60+
309+ Reviews
539+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the structures of the following two d-aldotetroses: Each of these compounds exists as a furanose ring, which is formed when the OH at C 4 attacks the aldehyde moiety. Draw each of the...
-
Consider the structures of the four d-aldopentoses (See the following figure). (a) Which d-aldopentose produces the same aldaric acid as d-lyxose? (b) Which d-aldopentoses yield optically inactive...
-
Consider the structures of the constitutional isomers, Compound A and Compound B (below). When treated with aqueous acid, Compound A undergoes isomerization to give a cis stereoisomer. In contrast,...
-
For each of the following tests, identify two different samples of people who would have the expertise to serve as subject matter experts (SMEs) for providing judgments regarding the content validity...
-
What advice would you offer an entrepreneur interested in launching a global business effort? (LO 2: Describe the nine principal strategies small businesses can use to go global; AACSB: Reflective...
-
Find the equations of the hyperbolas satisfying the given conditions. The center of each is at the origin. Passes through (8, 3), vertex (4, 0)
-
2. The encumbrance account of a governmental unit is debited when: a The budget is recorded b A purchase order is approved c Goods are received d A voucher payable is recorded
-
Peterson Company is preparing the annual financial statements dated December 31, 2010. Ending inventory information about the five major items stocked for regular sale follows: Required: Compute the...
-
Exercise 12-22 (Algorithmic) (LO. 2, 3,5) Yanni, who is single, provides you with the following information for 2022: Click here to access the exemption table. If required, round your answers to the...
-
Consider the AM signal s (t) = A c [1 + cos (2 m t)] cos (2 c t) produced by a sinusoidal modulating signal of frequency m . Assume that the modulation factor = 2, and the carrier frequency c is...
-
Could cash-to-cash cycle time be negative? How? Would that be good?
-
What would be some good inventory management performance measures for a fast-food company? A bicycle repair shop? A big-box retailer?
-
Probability-proportional-to-size (PPS) sampling is used by internal auditors to reach conclusions regarding monetary amounts. a. Describe the situation in which the application of PPS sampling is...
-
Exhibit 12: Average Credit Quality Ratios [1] Based on this information you can compare O&Rs financial ratios to the average debt rating ratios above to assess what O&Rs credit rating would be if it...
-
Which of the following statements about QuickBooks Bill Pay are correct? Select all that apply. You can configure QuickBooks Bill Pay to pay bills automatically when they're added to QuickBooks...
-
Ash purchases 500 shares of XYZ for $10/share. Ten months later, when the shares are trading at $15/share, they donate them to Caring Trust, a qualified charity. Three months after the donation is...
-
Bob gets a X = 60 on his psychology exam and a X = 56 on his Biology exam. Psych exam scores had a =50 and =10 while Bio exam scores had a =48 and =4. Both professors grade on a curve. 1 - For which...
-
1. Lucky Company's direct labor information for the month of February is as follows: Actual direct labor hours worked (AQ) 61,500 Standard direct labor hours allowed (SQ) 63,000 Total payroll for...
-
In 1995, there were 315 death sentences rendered by American juries. For the period from 1995 through 2014, the number of death sentences rendered by juries decreased by approximately 13 per year. If...
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
From what epoxide and what nucleophile colld each of the following compound be prepared Inppued? (Assums each is racemic.) C,H OH/H,O CH CH2 sodium azide
-
Suggest a Williamron other cynthosis, if one is possible, for each of the following compounds. If no Williamson ether synthesis is possible, explain why. (CH3)2CH---S---CH3
-
Show how the stereochemistry of the products will differ (if at all) when the following enantiomerically pure epoxide is hydrolyzed under acidic and basic conditions. D,C
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


