From what epoxide and what nucleophile colld each of the following compound be prepared Inppued? (Assums each
Question:
From what epoxide and what nucleophile colld each of the following compound be prepared Inppued? (Assums each is racemic.)
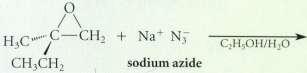
Transcribed Image Text:
C,H OH/H,O CH CH2 sodium azide
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
The strategy in this problem is to let the OH group originate fro...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Each of the following compounds has a nitrogennitrogen bond: N2, N2H4, N2F2. Match each compound with one of the following bond lengths: 110 pm, 122 pm, 145 pm. Describe the geometry about one of the...
-
Each of the following compounds is an aromatic compound bearing a substituent that we did not discuss in this chapter. Using the principles that we discussed in this chapter, predict the major...
-
Can the following compound be prepared via a Williamson ether synthesis? Explain your answer.
-
The portfolio of stock that comprises the ASX200 index is currently worth $5000. The continuously compounded interest rates on Australian government bonds is 1.5% per annum for each of the next five...
-
Assume initially that Demand and supply for premium coffees (one pound-bags) are in equilibrium. Now assume Starbucks introduces the world to premium blends so Demand rises substantially. Describes...
-
Assume Sharpie, a brand of Sanford LP, is planning to introduce a new executive pen that can be manufactured using either a capital-intensive method or a labor-intensive method. The predicted...
-
In research on artificial insemination of cows, a series of semen samples from each of six bulls was tested for the ability to produce conceptions (Snedecor and Cochran, 1989, Chapter 13, Section...
-
Review the data provided in Exercise 9-1. Metro Industries is considering the purchase of new equipment costing $1,200,000 to replace existing equipment that will be sold for $180,000. The new...
-
mathi.com Do Homework. Chrystina Hjelm MGMT 210 Financial Accounting - Jan 2020 - Online Homework: 8.6 - Homework: MyAccountingLabTM Homework 8 Score: 0 of 9 pts 3 of 12 (4 complete) E13-25 (similar...
-
On February 1, 2014, Punto Company purchased 95% of the outstanding common stock of Sara Company and 85% of the outstanding common stock of Rob Company. Immediately before the two acquisitions,...
-
What products are formed when gach of the following ethers reacts with concenffated aqueous HI? 2-ethoxy-2,3-dimethylbutane
-
Suggest a Williamron other cynthosis, if one is possible, for each of the following compounds. If no Williamson ether synthesis is possible, explain why. (CH3)2CH---S---CH3
-
A spherical vessel is filled with chemicals undergoing an exothermic reaction. The reaction provides a uniform heat flux on the inner surface of the vessel. The inner diameter of the vessel is 5 m...
-
Question TARIMAX MASTO Copper Explorations recently acquired the rights to mine a new site. Machinery, equipment and a truck were purchased to begin the mining operations at the site. Details of the...
-
Exercise 6 - 6 ( Algo ) The Town of Weston has a Water Utility Fund with the following trial balance as of July 1 , 2 0 2 3 , the first day of the fiscal year: During the year ended June 3 0 , 2 0 2...
-
The University of Cincinnati Center for Business Analytics is an outreach center that collaborates with industry partners on applied research and continuing education in business analytics. One of...
-
What is the correct answer to this? SQL QUESTION Sales Data for All Customers and Products Write a query that will return sales details of all customers and products. The query should return all...
-
Below are the jersey numbers of 11 players randomly selected from a football team. Find the range, variance, and standard deviation for the given sample data. What do the results tell us? 84 18 34 3...
-
What sources of equity (fund capital) do not-for-profit businesses have?
-
Tanaka Company's cost and production data for two recent months included the following: March April Production (units).........300................600 Rent.....................$1,800............$1,800...
-
Provide a reaction mechanism for step 1, formation of the imine, in Eq. 23.22. EtNH + H3C-C-CH3 acetone -HO [HC- NEt H3C-C-CH3 an imine (not isolated) H, Pt 30 psi EtOH NHET H3C-CH-CH3 (23.22)...
-
Provide reaction mechanisms for the following equations. (a) Eq. 23.17 (b) Eq. 23.18 (c) Suggest a reason why the reaction in Eq. 23.18 stops after two additions, and a third doesnt occur in high...
-
Using their solubilities in acidic or basic solution, design a separation of p-chlorobenzoic acid, p-chloroaniline, and p-chlorotoluene from a mixture containing all three compounds.
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...
-
International business and environment The MIR requires teams to gather current, or the most recently available, data on the markets people, economy, government, and technological status from online...
-
Consider the following stream of cash flows. The interest rate is 10%. 0 1 2 3 4 5 6 7 100 100 100 200 0 300 300 300 a) What is the value at time 0 of the cash flow stream? b) What is the value of...

Study smarter with the SolutionInn App


