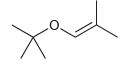
Can the following compound be prepared via a Williamson ether synthesis? Explain your answer.
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
No The Williamson ether synthesis employs a...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From what epoxide and what nucleophile colld each of the following compound be prepared Inppued? (Assums each is racemic.) C,H OH/H,O CH CH2 sodium azide
-
(a) Outline two methods for preparing isopropyl methyl ether by a Williamson ether synthesis. (b) One method gives a much better yield of the ether than the other. Explain which is the better method...
-
The following compound can react rapidly via an S N 1 process. Explain why this primary substrate will undergo an S N 1 reaction so rapidly. OTs
-
Solve exponential equation. Express irrational solutions in exact form and as a decimal rounded to three decimal places. 2 2x + 2 x - 12 = 0
-
The money aggregate M2 includes but is not limited to: A) Small denomination time deposits; B) Retail Money Market Mutual fund shares C) Savings Deposits and Money Market Deposit Accounts. D) M1 E)...
-
Simplify the given expressions. Express all answers with positive exponents. 4y-1/2 y 2/5
-
What is VPO?
-
Mass Spectrograph A mass spectrograph is used to measure the masses of ions, or to separate ions of different masses (see Section 27.5). In one design for such an instrument, ions with mass m and...
-
Check my work You have just been hired by FAB Corporation, the manufacturer of a revolutionary new garage door opening device. The president has asked that you review the company's costing system and...
-
Tidwell Corporation has 50,000 shares of $10 par value common stock outstanding. It declares a 10% stock dividend on December 1 when the market value per share is $16. The dividend shares are issued...
-
In an adiabatic compression of one mol of an ideal gas with C V ,m = 5/2 R, the temperature rises from 278 K to 450. K. Calculate q, w, H, and U.
-
Calculate H o R and U o R for the oxidation of benzene (g). Also calculate -U R.
-
What is meant by saying that a 1 confidence interval is a. Exact? b. Approximately correct?
-
How could civil engineers contribute to space debris management and cleanup?
-
In what ways can the principles of resilient infrastructure be applied to design urban systems capable of withstanding natural disasters, and how do these principles contribute to the overall safety...
-
What are local variables and global variables in Python?
-
When to use a tuple vs list vs dictionary in Python? Explain some benefits of Python
-
What is Lambda Functions in Python? How do I modify a string in python?
-
Lomax Corporation manufactures hiking boots. For the coming year, the company has budgeted the following costs for the production and sale of 40,000 pairs of boots: Instructions a. Compute the sales...
-
Use of the contraceptive Depo Provera appears to triple women's risk of infection with chlamydia and gonorrhea , a study reports today. An estimated 20 million to 30 million women worldwide use Depo...
-
The DNA of sea urchins contains about 32% A. What percentages of the other three bases would you expect in sea urchin DNA? Explain.
-
The codon UAA stops protein synthesis. Why does the sequence UAA in the following stretch of mRNA not cause any problems? -GCA-UUC-GAG-GUA-ACG-CCC-
-
Which of the following base sequences would most likely he recognized by a restriction (endonuclease? Explain. (a) GAATTC (b) GATTACA (c) CTCGAG
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


