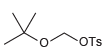
The following compound can react rapidly via an S N 1 process. Explain why this primary substrate
Question:
Transcribed Image Text:
OTs
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (17 reviews)
When the leaving grou...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following compound can become protonated on any of the three nitrogen atoms. One of these nitrogens is much more basic than the others, however. (a) Draw the important resonance forms of the...
-
The following compound can?t be synthesized using the methods discussed in this chapter. Why not?
-
Explain why this primary halide reacts very rapidly under condition that is favorable for the SN1 mechanism: CH3 O CH2 Cl
-
Hussein Hage has just approached a venture capitalist for financing for his new restaurant, Bistro Sally. The lender is willing to loan Bistro Sally Inc. $240,000 at a high-risk interest rate of 9%....
-
What is the key feature of the Gramm-Leach-Bliley Financial Services and Modernization Act of 1999?
-
In Exercises find an equation of the tangent line to the graph of the function at the given point. y = (sin x)x, 2
-
Rolling a Die You roll a die. Find each probability. (a) Rolling a 5 or a number greater than 3 (b) Rolling a number less than 4 or an even number (c) Rolling a 2 or an odd number
-
Winter Lips produces a lip balm used for cold-weather sports. The balm is manufactured in a single processing department. No lip balm was in process on May 31, and Winter Lips started production on...
-
Green Company sells its product for $11000 per unit. Variable costs per unit are: manufacturing, $6700; and selling and administrative, $140. Fixed costs are: $31200 manufacturing overhead, and...
-
Event A: Randomly select a male psychology major. Event B: Randomly select a psychology major who is 20 years old. Determine whether the events are mutually exclusive. Explain your reasoning.
-
As shown in Table 16.2, $1,000 invested at 10 percent compound interest will grow into $1,331 after three years. What is the present value of $2,662 in three years if it is discounted back to the...
-
Angela puts $1,000 in a savings account that pays 3 percent per year. What is the future value of her money one year from now? a. $970. b. $1,000. c. $1,003. d. $1,030.
-
Write the general form of an equation of state as: Z=1+ Z rep () Z attr (T, ) where Z rep () represents contributions from repulsions, and Z attr (T, ) represents contributions from attractions. What...
-
4. (7%) Problem 4: Consider a 570 nm light falling on a single slit of width 1.1 m. Randomized Variables =570 nm w=1.1 um Forbes, David david.forbes@doane.edu @theexpertta.com - tracking id:...
-
(b) The following results are obtained in a double-slit experiment using light from a helium-neon gas laser: Width of 15 fringes = 3.0 cm Separation of slits = 1.5 mm Slit-to-screen distance = 2.5 m...
-
Read the mini-case, Ben and Jerry's Corporate Activism, and answer the following question: What are the pros and cons of Ben and Jerry's political activism when compared to other corporate political...
-
TOPIC : PROBLEMATIZATION - SECOND CURVE THINKING 1. What is second curve thinking?( a More in depth explanation ) 2. What are the implicit assumptions of second curve thinking? ( a More in depth...
-
I think the Power Distance measure in Hofstede's model (Hofstede Insights, n.d.) is particularly interesting.I led divisions in the U.S., New Zealand, and Thailand.Those three countries represented a...
-
Use the graph of y = f(x) to determine if f is one-to-one. Does f have an inverse? y = f(x) 3
-
Anna, a high school counselor, devised a program that integrates classroom learning with vocational training to help adolescents at risk for school dropouts stay in school and transition to work...
-
What products would you obtain from reaction of 1-penlanol with the following reagents? (a) PBr3 (b) SOCl2 (c) CrO3, H20, H2SO4 (d) PCC
-
How would you prepare the following compounds from 2-phenylethanol? More than one step may be required. (a) Styrene (PhCH = CH2 (b) Phenyl acetaldehyde (PhCH2CHO) (c) Phenyl acetic acid (PhCH2CO2H)...
-
How would you prepare the following compounds from 1-phenylethanol? More than one step may be required. (a) Acetophenone (PhCOCH3) (b) Benzyl alcohol (c) m-Bromobenzoic acid (d) 2-Phenyl-2-propanol
-
A company sells two products. Assuming the same sales mix as shown below, how many units of Product A must be sold to breakeven? Product A Product B Total Units 100,000 150,000 250,000 Sales $300,000...
-
South Sea Baubles has the following (incomplete) balance sheet and income statement. BALANCE SHEET AT END OF YEAR (Figures in $ millions) Assets 2015 2016 Liabilities and Shareholders' Equity 2015...
-
When the investor pays $100,000 to acquire 40% of a company's outstanding voting shares at a time when the fair value of the company's net assets are $175,000, the resulting goodwill amount is...

Study smarter with the SolutionInn App


