Consider the three compounds shown below and then answer the questions that follow: a) Which two compounds
Question:
a) Which two compounds are constitutional isomers?
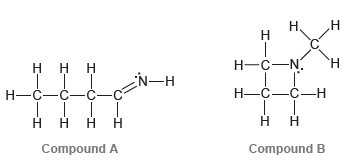
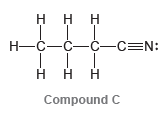
b) Which compound contains a nitrogen atom with trigonal pyramidal geometry?
c) Identify the compound with the greatest number of σ bonds.
d) Identify the compound with the fewest number of σ bonds.
e) Which compound contains more than one π bond?
f) Which compound contains an sp2-hybridized carbon atom?
g) Which compound contains only sp3-hybridized atoms (in addition to hydrogen atoms)?
h) Which compound do you predict will have the highest boiling point? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





