Consider the two protons highlighted in the following compound: Do you expect these protons to be equivalent,
Question:
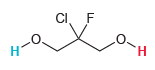
Do you expect these protons to be equivalent, or is one proton more acidic than the other? Explain your choice.
Transcribed Image Text:
CI. F TH. Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Both protons are the same distance fr...View the full answer

Answered By

Krishnavendra Y
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagiarism), well-researched and critically analyzed papers.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Do you expect the following compound to be chiral? Explain your answer (consider whether this compound is super-imposable on its mirror image).
-
Consider the following depictions of two atoms, which have been greatly enlarged so you can see the subatomic particles. a. How many protons are present in atom A? b. What is the significance of the...
-
Consider the following compound: (a) How many signals do you expect in the 1 H NMR spectrum of this compound? (b) Rank the protons in terms of increasing chemical shift. (c) How many signals do you...
-
Assume you have just been hired as a business manager of PizzaPalace, a regional pizza restaurant chain. The companys EBIT was $120 million last year and is not expected to grow. PizzaPalace is in...
-
Look at the Focus on Ethics box ("How Fair Is Check Into Cash") in Chapter 5 of the textbook. These, businesses quote an interest rate of 15% to loan customers (most of whom are fairly...
-
Use Lagrange multipliers to find the dimensions of a rectangular box of maximum volume that can be inscribed (with edges parallel to the coordinate axes) in the ellipsoid = 1. + 2 +
-
Design an experiment. A universitys Department of Statistics wants to attract more majors. It prepares two advertising brochures. Brochure A stresses the intellectual excitement of statistics....
-
At December 31, 2013, Obermeyer Imports reported the following information on its balance sheet. Accounts receivable $250,000 Less: Allowance for doubtful accounts 15,000 During 2014, the company had...
-
Score: 0 of 15 Suppose the Baseball Hall of Fame in Cooperstown, New York, has approached Alley - Carde wth a special Ordet. The Hall of Fame wishes to purchase 57,000 baseball card packs for a...
-
Given a database of the results of an election, find the number of seats won by each party. There are some rules to going about this: There are many constituencies in a state and many candidates who...
-
Consider the structure of 2,3-dichloropropanoic acid: This compound has many constitutional isomers. (a) Draw a constitutional isomer that is slightly more acidic and explain your choice. (b) Draw a...
-
Identify which of the following compounds is more acidic. Explain your choice. C=C H--
-
A three-phase, four-wire system operating with a 208-V line voltage is shown in Fig. 12.71. The source voltages are balanced. The power absorbed by the resistive wyeconnected load is measured by the...
-
What should be the equivalent units of production for (1) Dept M and (2) Dept. P? Can you please show the solutions and answer. Thanks Problem 1 Lee Gon Mfg. Co has its product processed in two...
-
Moullierat Mfg. is considering a rights offer. The company has determined that the ex-rights price will be $95. The current price is $102 per share, and there are 24 million shares outstanding. The...
-
This question involves hypothesis testing. The following numbers will help you answer these questions. The random variable Z ~N(0, 1) is standard normal. P(Z >1.28).1 P(Z1.65) .05 P(Z1.96) .025 P(Z...
-
Human service organizations require strong and effective leadership. Understanding what qualities make up an effective leader and how these qualities can be cultivated is of critical importance for...
-
18. What is the name of the heat treatment performed on a cold worked sample? 19. What is the percent coldwork of a sample with an initial thickness of 11mm and a final thickness of 7mm? 20. Which...
-
How many liters each of 8% and 20% hydrogen peroxide should be mixed together to obtain 8 L of 12.5% solution?
-
A company has the following incomplete production budget data for the first quarter: In the previous December, ending inventory was 200 units, which was the minimum required, at 10% of projected...
-
Propose structures for each of the following products derived from oxirane (ethylene oxide): (a) (b) (c) (d) (e) Methyl Cellosolve cat. HA, CH1002 EtOH Ethyl Cellosolve H2O
-
Give bond-line formulas and appropriate names for all of the alcohols and ethers with the formulas (a) C3H8O and (b) C4H10O.
-
Provide a mechanistic explanation for the following observation. MeONa MeOH MeO
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


