Question: Cyclopropane (shown below) has only one constitutional isomer. Draw a condensed structure of that isomer. -. - CH2 H2
Cyclopropane (shown below) has only one constitutional isomer. Draw a condensed structure of that isomer.
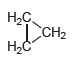
-. - CH2 H2
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
H ... View full answer

Get step-by-step solutions from verified subject matter experts


