Deduce the structure of a compound with molecular formula C 6 H 14 O 2 that exhibits
Question:
Deduce the structure of a compound with molecular formula C6H14O2that exhibits the following IR,1H NMR, and13C NMR spectra.
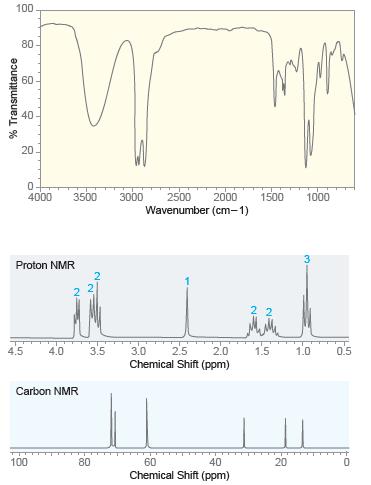
Transcribed Image Text:
100 80 60 40 20 4000 3500 3000 2500 2000 1500 1000 Wavenumber (cm-1) Proton NMR 4.5 2.0 Chemical Shift (ppm) 4.0 3.5 3.0 2.5 1.5 1.0 0.5 Carbon NMR 100 80 60 40 20 Chemical Shift (ppm) % Transmittance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Deduce the structure of a compound with molecular formula C 9 H 10 O 2 that produces the following 1 H NMR spectrum and 13 C NMR spectrum: Proton NMR 10 Chemical Shift (ppm) Carbon NMR - 128.4 128.8-...
-
The IR spectrum of a compound with molecular formula C5H8O was obtained in CCl4 and is shown in Figure 13.42. Identify the compound. Wavelenga qum) 15 16 14 3600 340) 3800 3300 3000 280K 2600 2400...
-
The 1 H NMR spectrum of a compound with molecular formula C 7 H 15 C l exhibits two signals with relative integration 2 : 3. Propose a structure for this compound.
-
In which section do you create VLAN on Cisco WLC ? ? Layer 3 3 Section Layer 2 2 Section Security Section RF Section
-
After reading the information presented in this chapter and other sources, write a one-page paper that describes net neutrality. Describe how a non-neutral network will impact all businesses.
-
In Exercises find the indefinite integral. Is sec5 x tan x dx
-
3. Why does the return on equity differ between Company A and Company C? Is this difference attributable to operating performance? Which better reflects operating performance, return on assets or...
-
The following information was abstracted from the accounts of the General Fund of the City of Rome after the books had been closed for the fiscal year ended June 30, 2017. There were no transfers...
-
CALCULATOR FULL SCREEN PRINTER VERSION BACK NEX Exercise 192 Prepare the necessary closing entries based on the following selected accounts. (Credit account titles are automatically indented when the...
-
For a standardized normal distribution, determine a value, say zo such that the following probabilities are satisfied. a. P(0
-
Because investment and capital goods are paid for with savings, higher savings rates reflect a decision to consume fewer goods for the present in order to be able to invest in more goods for the...
-
Look back at Figure 2, which shows the inverse relationship between ticket prices and game attendance at Gigantic State University. (a) Interpret the meaning of both the slope and the intercept. (b)...
-
When should liabilities for each of the following items be recorded on the books of a company? (1) Warranty, (2) Acquisition of goods by purchase on credit, and (3) Profit-sharing bonus.
-
Financial Statement Items Identify the financial statement (or statements) in which each of the following items would appear: income statement (IS), statement of stockholders' equity (SSE), balance...
-
Recall from Chapter 4 that Tiger Stripe Copy Center is a small business located near a large university campus. Tiger Stripe Copy offers a range of services to walk-in customers, including passport...
-
Accounting Processes Identify the following processes as either measuring or communicating. a. Prepare financial statements for the entity b. Identify relevant economic activities of the entity c....
-
To estimate future values of the cost indices, one is tempted to assume that the average value for the year occurred at midyear (June 30-July 1) and that the linear fit to the recent data can be...
-
Reston Manufacturing Corporation produces a cosmetic product in three consecutive processes. The costs of Department | for May 2016 were as follows: Department | handled the following units during...
-
Simplify the expression without a calculator. 3
-
Prepare a stock card using the following information A company is registered for GST which it pays quarterly, assume GST was last paid on the 30th of June 2019. It uses weighted average cost...
-
Name the following alkenes: ) CH2H2CH {c) CH-CH (a) H (b) -CH CHCH2CH3 CH H%3H2H H3C (9) H%3D%3H3 (e) CH (d) C=C 23DCHCHCH CH CH CH CHCH2CH2
-
Ocimene is a triene found in the essential oils or many plants. What is its IUPAC name, including stereo chemistry? Ocimene
-
? - Farnesene is a constituent of the natural wax found on apples. What is its IUPAC name, including stereo chemistry? a-Farnesene
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...

Study smarter with the SolutionInn App


