Determine the hybridization state of each carbon atom in the following compounds: a. b. , C=c=c=C
Question:
a.
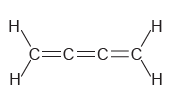
b.
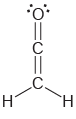
Transcribed Image Text:
Н, н C=c=c=C Н н || Н `H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
a b ...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the hybridization state of each carbon in the following Compounds: (b) Formaldehyde (H2C=O) (c) Ketene (H2 C=C=O) (d) Propane (CH3CH=CH2) (e) Acetone [(CH3)2C=O] (f) Acrylonitrile (CH2=CHC CPN)
-
Give the hybridization state of each carbon in the following compounds: (b) Formaldehyde (H2C==O) (c) Ketene (H2C==C==O) (d) Propene (CH3CH==CH2) (e) Acetone [(CH3)2C==O] (f) Acrylonitrile (CH2==CHCN)
-
Below are the structures of two common over-the-counter pain relievers. Determine the hybridization state of each carbon atom in these compounds: a. b. TH. H H H. .C. TH. .C: .. `H Acetylsalicyclic...
-
In seawater, the pressure p is related to the depth d according to 33p - 18d = 495 where d is in feet and p is in pounds per square inch. (a) Solve this equation for p in terms of d. (b) The Titanic...
-
"You know, we recently had a soft drink product (an exotic berry seltzer line) go through one of those simulated test markets, and it was a disaster. The new products people forgot completely about...
-
In Exercises evaluate the definite integral. 41/3 sec d
-
A combination is an ordered arrangement of objects.
-
Presented below are the consolidated work paper balances of Bush, Inc., and its subsidiary, Dorr Corporation, as of December 31, 2016 and 2015: Additional information: a. On January 20, 2016, Bush,...
-
Question 3 10 pts Presented below are a list of accounts from the balance sheet or income statement. Assume that all accounts have normal balances according to whether the account is increased by a...
-
The Westerbeck Company manufactures several models of automatic washers and dryers. The projected requirements over the next year for their washers follow. Current inventory is 100 units. Current...
-
Identify the reagents you would use to accomplish the following transformation. CI
-
At year-end 2009, total assets for Shome Inc. were $1.2 million and accounts payable were $375,000. Sales, which in 2009 were $2.5 million, are expected to increase by 25 percent in 2010. Total...
-
Is all compensation paid to an employee deductible? Discuss the circumstances in which employee compensation cannot be deducted.
-
5) A frictionless rod of length L rotates counterclockwise in the with constant angular speed w at an angle a to the z axis. A bead of mass m, free to slide on the rod, leaves the origin with initial...
-
1) Louisa is a corn farmer in Illinois. She anticipates a harvest in August of 3 million bushels of yellow corn. Today is May. Louise plans to hedge her sale of corn in August using corn futures...
-
2. DETAILS MY NOTES In a statistical test, we have a choice of a left-tailed test, a right-tailed test, or a two-tailed test. Is it the null hypothesis or the alternate hypothesis that determines...
-
2. The model of a two-story building shown in Figure 2. The girders are assumed to be rigid, and the columns have flexural rigidities EI and EI2, with negligible masses. The stiffness of each column...
-
Prepare journal entries to record these transactions. (List all debit entries before credit entries. Credit account titles are automatically indented when amount is entered. Do not indent manually....
-
Sketch a graph of y = f(x). f(x) = 2(3)
-
According during to the IRS, individuals filing federal income tax returns prior to March 31 received an average refund of $1,088 in 2018. Consider the population of "last-minute" filers who mail...
-
Draw an energy diagram for a reaction with Keq = 1. What is the value of G in this reaction?
-
The addition of water to ethylene to yield ethanol has the following thermodynamic parameters: (a) Is the reaction exothermic or endothermic? (b) Is the reaction favorable (spontaneous) or...
-
When a mixture of methane and chlorine is irradiated, reaction commences immediately. When irradiation is stopped, the reaction gradually slows down but does not stop immediately. Explain.
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


