Below are the structures of two common over-the-counter pain relievers. Determine the hybridization state of each carbon
Question:
a.
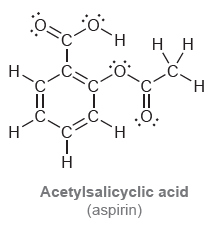
b.
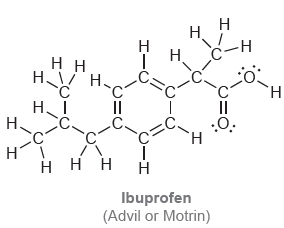
Transcribed Image Text:
TH. H H H. .C. TH. .C: .Č. `H Н Acetylsalicyclic acid (aspirin) Dーエ Н Н. н. нн Н. Н. H. Н I II II :O. Н. C. н ннн н Ibuprofen (Advil or Motrin)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
All carbon atoms in this molecule aresp 2 hybridized ex...View the full answer

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the hybridization state of each carbon atom in the following compounds: a. b. , C=c=c=C || `H.
-
Identify the hybridization state and geometry of each carbon atom in the following compounds: a. b. c. -OEJ- -CEC-C . .C. H. 1 H' .
-
Describe the hybrid orbitals used by each carbon atom in the following molecules: a. b. C-C-C-OH
-
Use normal job-order costing to calculate the balance in the ending Work-in-Process account and the Cost of Goods Sold account on 31 December 20X3 after any necessary adjustment(s). Sweet Memories...
-
When the United Kingdom chose not to participate in the European Currency Union, it gave itself additional domestic monetary policy independence. How is this so?
-
Agnes passes away in 2022 and leaves her daughter, Sam, 100 shares of stock. Agnes purchased the stock for $1,000 over twenty-five years ago. It was worth $10,000 on the date of Agnes death. Sam...
-
Writing Is there a relationship between independence and mutual exclusivity? To decide, find examples of the following, if possible. (a) Describe two events that are dependent and mutually exclusive....
-
According to its directional distribution, solar radiation incident on the earth's surface may be divided into two components. The direct component consists of parallel rays incident at a fixed...
-
When direct labor costs are recorded in a job costing 12 0.50 JIS .a. Direct Labor and Indirect Labor are debited and Factory Wages Payable is credited O b. Factory Wages Payable is debited and Work...
-
Motion of the sliding block P in the rotating radial slot is controlled by the power screw as shown. For the instant represented, = 0.1 rad/s, = 0.04 rad /s2, and r = 300 mm. Also, the screw turns...
-
Consider the reaction below. The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the reaction mixture. The sodium iodide is not consumed by the reaction and...
-
Identify the number of sp 3 -hybridized carbon atoms in the following compound: (CH 3 ) 2 C = CHC(CH 3 ) 3
-
What objections to the real business cycle model have been raised?
-
Two firms are bidding for a $100 million contract in an all-pay auction. The bidding continues over many rounds, and each firm must incur a non-recoverable cost equal to 1% of the total value of the...
-
Illustrate the different steps required for the insertion of 58 followed by the deletion of 40 in the following AVL tree. 55 40 50 65 60 60 57 70 70
-
3 undamaged, the suit is returned to the customer. If either (or both) of the parts is (are) damaged, the suit goes to customer relations (Server 5). Assume that all travel times are negligible (0),...
-
Saginaw Incorporated completed its first year of operations with a pretax loss of $677,500. The tax return showed a net operating of $826,500, which the company will carry forward. The $149,000...
-
General Average Problem You've just learned about the concept of general average. Try applying it to the following hypothetical: A cruise ship docks close to Rome for three days, giving the...
-
Find a linear function f and an exponential function g whose graphs pass through the two given points. (0,4), (1,8)
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
Benzyl chloride can be converted into benzaldehyde by treatment with nitro methane and base. The reaction involves initial conversion of nitro methane into its anion, followed by SN2 reaction of the...
-
Reduction of 2-butanone with NaBH4 yields 2-butanol. Is the product chiral is it optically active? Explain.
-
Reaction of (S)-3-methyl-2-pentanone with methyl magnesium bromide followed by acidification yields 2, 3-dlmethyl-2-pentanol. What is the stereo chemistry of the product? Is the product optically...
-
essential con Example 15: Mr. Sunil Mukharjee has estimated probable under different macroeconomic conditions for the following three stocks: Stock Current Price (Rs.) Rates of return (%) during...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...

Study smarter with the SolutionInn App


