Determine whether each of the following disaccharides is a reducing sugar. (a) (b) (c) CH,OH HO CH,OH
Question:
Determine whether each of the following disaccharides is a reducing sugar.
(a)
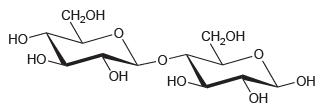
(b)
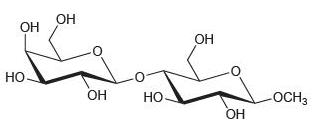
(c)
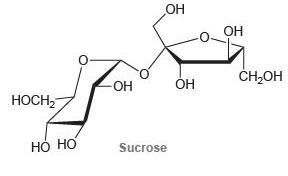
Transcribed Image Text:
CH,OH HO CH,OH Но. ОН но. -OH OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a Yes one of the anomeric pos...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each of the following transport systems is uniport, symport, or antiport. Which systems are active transport systems? (a) Glucose transporter in erythrocytes (b) Valinomycin (c)...
-
Determine whether each of the following compounds is a terpene. (a) (b) (c) (d)
-
Determine whether each of the following reactions proceeds via an S N 1 or S N 2 mechanism and then draw the product(s) of the reaction: (a) (b) (c) (d) (e) (f) .? , Br cP HMPA
-
The main ingredient of vinegar is acetic acid (HAc) that will dissociate into acetate (Ac - ) and hydrogen ion (H + ) in water: HAc < = > Ac - (aq) + H + (aq) . K HAc(room temp) = 1.8x10 -5 ....
-
Mason Fender is a competitor of Matthews Fender from Exercise E23-19. Mason Fender also uses a standard cost system and provides the following information: Static budget variable overhead...
-
According to Example 14-1, the mass percent ethanol in a particular aqueous solution is less than the volume percent in the same solution. Explain why this isalso true for all aqueous solutions of...
-
Brain and body. The correlation between body weight and brain weight is r = 0.86. How well does body weight explain brain weight for mammals? Give a number to answer this question, and briefly...
-
1. Which of the following statements regarding aggregate planning is true? A) In a pure level strategy, production rates or work force levels are adjusted to match demand requirements over the...
-
Question 31 (2 points) Which of the following is not true of absorption costing? Question 31 options: It allows companies to possibly hide fixed costs in ending inventory Direct fixed manufacturing...
-
Evaluate Canam Groups need for better internal communication. What was driving the need for an intranet? How did using Facebook for an intranet come about? Is that a good model for every company to...
-
When d-glucose undergoes a Wohl degradation followed by a Kiliani-Fischer chain-lengthening process, a mixture of two epimeric products are obtained. Identify both epimers.
-
Draw the structure of the product obtained when the following disaccharide is treated with NaBH 4 in methanol. CH- CH- - -
-
Atlanta Company is preparing its manufacturing overhead budget for 2012. Relevant data consist of the following. Units to be produced (by quarters): 10,000, 12,000, 14,000, 16,000. Direct labor: Time...
-
2.11.2Project:Performance Task: The Parallax Problem Project Geometry Sem 1 (S3537251) Julio Duenas Points possible:120 Date: ____________ The Scenario:You're looking for a sponsor to pay for you to...
-
If the most common treatment of assigning overapplied overhead was used, the final balance in Cost of Goods Sold would have been * (1 Point) At the end of the last fiscal year, BREAD Company had the...
-
Angelina received new word processing software for her birthday. She also received a cheque with which she intends to purchase a new computer. Angelina's UNILUS Professor assigned a paper due in two...
-
At date t, the portfolio P to be hedged is a portfolio of Treasury bonds with various possible maturities. Its characteristics are as follows: Value YTM MD Convexity $1,450 6% 4.25 55 We consider...
-
A playground merry-go-round with an axis at the center (radius R = 1.3 m and rotational inertia | = 1.2 x 103 kgm2) is initially rotating at angular velocity w = 0.21 rad/s clockwise). A girl of mass...
-
Define the three levels of fair values. Which one is the most difficult to audit? Why? Audits of which asset accounts are most likely affected by these most difficult fair values?
-
Solve the relation Exz:Solve therelation ne %3D
-
(a) Optically active 2-bromobutane undergoes racemization on treatment with a solution of KBr. Give a mechanism for this racemization. (b) In contrast, optically active butan-2-ol does not racemize...
-
For each pair of compounds, predict which compound has the higher boiling point. Check Table 6-2 to see if your prediction was right, then explain why that compound has the higher boiling point. (a)...
-
Predict the products of E1 elimination of the following compounds. Label the major products. (a) (b) (c) , , Br (CHC-CH CH Br
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


