Draw a mechanism for each of the following reactions: (a) (b) 1) EtMgBr 2) - I
Question:
(a)
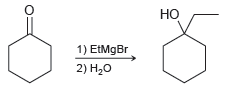
(b)
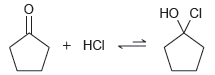
Transcribed Image Text:
Но 1) EtMgBr 2) Н-о Но I + HCI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a ...View the full answer

Answered By

Amit Kumar
I am a student at IIT Kanpur , which is one of the prestigious colleges in INDIA.
Cleared JEE Advance in 2017.I am a flexible teacher because I understand that all students learn in different ways and at different paces. When teaching, I make sure that every student has a grasp of the subject before moving on.
I will help student to get the basic understanding clear. I believe friendly behavior with student can help both the student and the teacher.
I love science and my students do the same.
4.90+
44+ Reviews
166+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a mechanism for each of the following transformations: a. b. c. Br Dilute H Dilute HBr Br, Br,
-
Suggest a mechanism for each of the following reactions that accounts for both products. H,so, CH,CH CHCH,OH HBr CH,CH CHCH BrCH,CHCH CH2 (84%) Br (16%)
-
Draw the mechanism for each of the following reactions: a. b. c. NaOMe CI NaOEt. Br
-
The adjusted trial balance for Sweet Home Catering, Inc., is presented below. Prepare the income statement and statement of retained earnings for Sweet Home Catering, Inc., for the month ended March...
-
On February 12, Addison, Inc. purchased 6,000 shares of Lucas Company at 22 per share plus a 240 brokerage fee. On August 22, Lucas paid a 0.42 dividend per share. On November 10, 4000 shares of...
-
Evaluate the following improper integrals whenever they are convergent. 00 So 0 -3x dx
-
The specification for the weight of a chemical in a compound is .05. If the standard deviation of the weighing scales is .02, is the process considered capable? LO.1
-
HealthSouth Corporation claims to be . . . the nations largest owner and operator of inpatient rehabilitation hospitals in terms of revenues, number of hospitals, and patients treated and discharged....
-
A local government received $5,000,000 from the State for its share of the States income taxes. The resources received by the local government would be reported on the local governments...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Identify the reagents necessary to achieve each of the following transformations: (a) (b) (c) (d) (e) (f) HO H.
-
Draw a mechanism for each of the following reactions: (a) (b) HO 1) EtMgBr 2) H20
-
Repeat Exercise 15 for 500 g of plutonium-241, which decays according to the function A(t) = A 0 e -0.053t , where t is time in years. Exercise 15 (a) 4 yr, (b) 8 yr, (c) 20 yr. (d) Find the...
-
(AVR) PR=IAVR=1R = (power dissipated by a resistor) (28.12) R
-
As a manager and an entrepreneur, you will face a new challenge - business venture structured on the theory of the firm. You are opening a restaurant in your selected town in the State of NY (please...
-
Install on ubuntu , please provide a screenshot for each step 1)How to install base64 on ubuntu 2)What kind of analysis is performed by Cuckoo? How to install Cuckoo on ubuntu?
-
rt a letter to Rose McBride. Writing Plan - Refusal to a Request Rubric Buffer: Start with a neutral statement on which both reader and writer can agree, such as a compliment, appreciation, a quick...
-
FACTS: The Budvar Company sells parts to a foreign customer on December 1, Year 1, with payment of 20,000 crowns to be received on March 1, Year 2. Budvar enters into a forward contract (with a...
-
Complete each statement. If the two lines forming a system have different slopes, the system has (no / one / infinitely many) solution(s).
-
For the vector whose polar components are (Vr = 1, Vθ = 0), compute in polars all components of the second covariant derivative Vα;μ;ν. To find...
-
Write structural formulas for at least three constitutional isomers with the molecular formula CH3NO2. (In answering this question you should assign a formal charge to any atom that bears one.)
-
Write the resonance structure that would result from moving the electrons in the way indicated by the curved arrows. H2N
-
Consider the following compounds and decide whether the bond in them would be ionic or covalent. (a) KCl (b) F2 (c) PH3 (d) CBr4
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


