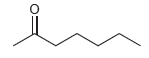
Draw a resonance structure of the compound shown below, called 2-heptanone, which is found in some kinds
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw a resonance structure of the compound below, which was isolated from the fruits of Ocotea corymbosa, a native plant of the Brazilian Cerrado.
-
Draw a resonance structure of the acetonitrile anion, :CH 2 C N, and account for the acidity of nitriles.
-
The compound benzenc has only one type of carbon-carbon bond, and this bond has a length intermediate between that of a single bond and a double bond. Draw a resonance structure of benzene that,...
-
Coffin Corporation appropriately uses the installment-sales method of accounting to recognize income in its financial statements. The following information is available for 2014 and 2015....
-
Walters Corporation sells radios for $50 per unit. The fixed costs are $420,000 and the variable costs are 60% of the selling price. As a result of new automated equipment, it is anticipated that...
-
Find the Jacobian for the indicated change of variables. If then the Jacobian of x, y, and z with respect to u, v, and w is x = u(1 - v), y = uv(1 - w), z = uvw a(x, y, z) d(u, v, w)
-
P 21-7 Enterprise fund statement of cash flows Caleb County had a beginning cash balance in its enterprise fund of $714,525. During the year, the following transactions affecting cash flows occurred:...
-
During its first year of operations, Macks Plumbing Supply Co. had net sales of $3,250,000, wrote off $27,800 of accounts as uncollectible using the direct write-off method, and reported net income...
-
Markus Company's common stock sold for $5.25 per share at the end of this year. The company paid a common stock dividend of $0.63 per share this year. It also provided the following dato excerpts...
-
1. Calculate the internal growth rate and sustainable growth rate for S&S Air. What do these numbers mean? 2. S&S Air is planning for a growth rate of 12 percent next year. Calculate the EFN for the...
-
Consider a gas mixture in a 1.50-dm 3 flask at 22.0C. For each of the following mixtures, calculate the partial pressure of each gas, the total pressure, and the composition of the mixture in mole...
-
A mixture of H 2 and NH 3 has a volume of 139.0 cm 3 at 0.00C and 1 atm. The mixture is cooled to the temperature of liquid nitrogen, at which ammonia freezes out and the remaining gas is removed...
-
Solve the following simultaneous equations. xy = 2 x + y = 3
-
Listed in the accompanying table are waiting times (seconds) of observed cars at a Delaware inspection station. The data from two waiting lines are real observations, and the data from the sir line...
-
Franklin Prepared Foods (FPF) sells three varieties of microwaveable meals with the following prices and costs: Variable Cost Fixed Cost per Meat Fish Vegetarian Entire firm Selling Price per Case: $...
-
Isabella is a 14-year-old Hispanic bisexual female who has come into the Department of Child Safety (DCS) care due to neglect. Isabella's mother, Martina, is 35 years old, a single mother, has an...
-
Jeff is able to ride a bicycle although he hasn't ridden one for a few years, thanks to his: ( A ) procedural memory ( B ) episodic memory C ) semantic memory ( D ) cognitive memory
-
1. Allen Young has always been proud of his personal investment strategies and has done very well over the past several years. He invests primarily in the stock market. Over the past several months,...
-
A wholesaler supplies college t-shirts to three college bookstores: A, B, and C. The wholesaler recently shipped a total of 800 t-shirts to the three bookstores. Twice as many t-shirts were shipped...
-
A red card is illuminated by red light. What color will the card appear? What if its illuminated by blue light?
-
In one industrial synthesis of ethanol, ethene is first dissolved in 95% sulfuric acid. In a second step water is added and the mixture is heated. Outline the reactions involved. Discuss.
-
The reaction of bromine with cyclohexene involves anti addition, which generates, initially, the diaxial conformation of the addition product that then undergoes a ring flip to the diequatorial...
-
Propose a mechanism that explains formation of the products from the following reaction, including the distribution of the products as major and minor. cat 2 Minor Major
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


