Draw all significant resonance structures for each of the following compounds: Testosterone Estradiol (Female sex hormone) (Male
Question:
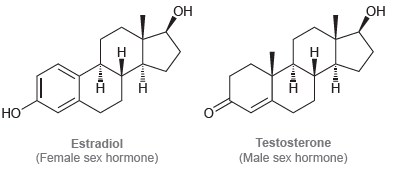
Transcribed Image Text:
ОН Он НО Testosterone Estradiol (Female sex hormone) (Male sex hormone)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
HO He OH O...View the full answer

Answered By

Tamil Elakkiya Rajendran
I'm currently involved in the research in the field of Biothermodynamics, Metabolic pathway analysis and computational Biology. I always prefer to share my knowledge whatever I have learnt through my degree whenever time permits.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the structure of ozone: Ozone is formed in the upper atmosphere, where it absorbs short-wavelength UV radiation emitted by the sun, thereby protecting us from harmful radiation. Draw all...
-
Cycloserine is an antibiotic isolated from the microbe Streptomyces orchidaceous. It is used in conjunction with other drugs for the treatment of tuberculosis. a) What is the molecular formula of...
-
Draw significant resonance structures for the following compound:
-
Compute for the fringe benefits tax due in the following scenarios. During 201A, Alpha Corporation gave the following fringe benefits to its employees: Salaries to rank and file employees P1,000,000...
-
Define international business and discuss the advantages and challenges of globalization. Discuss two (2) advantages and two (2) challenges of an international business.
-
Briefly describe the contributions of the four individuals identified in the preceding question.
-
7. What more could boards of directors and shareholders do to ensure that managers pursue long-term value creation?
-
The following information was taken from the 2009 financial statements of pharmaceutical giant Merck and Co. All dollar amounts are in millions. Retained earnings, January 1, 2009 ... $43,698.8...
-
You have spent two years working as an auditor. In that time, you have come across a number of errorsin performing bank reconciliations. Outlined below are some of them: 1. An unreconciled item of...
-
Which organizutional structure issue is most apparent at Authentic Brew based on the Mating ent Pckai Deptent Shpng Denartment Tap Ro acatis Selesa Prmtn Pursteng Bing ewing Coordination of...
-
Propose a plausible synthesis for each of the following transformations: a. b.
-
As the baby boom generation (born between 1946 and 1964) ages, which of the following is a likely outcome? a). A movement to the right in the demand for nursing home beds b). A movement to the left...
-
The line segment CD is a diameter of the circle centre (2a, 5a). Given D has coordinates (3a, 7a), find the coordinates of C.
-
While sovling y' = Ky(Ay), we used the fact that 1 y(1) 1 1 - y y-A Use partial fraction decomposition (and a bit of algebra) to show that this is true. 1) Use partial fractions. Do not merely...
-
You are fluent in three languages. In terms of your strengths, this is an example of what? a. Personal brand b. Vocation c. Experience d. Competency
-
Miller Company's contribution format income statement for the most recent month is shown below: Sales (45,000 units) Variable expenses Contribution margin Fixed expenses Net operating income...
-
Find f''(x). f(x) = (x+8) 5 f''(x) =
-
How does the market play into business financials? 2- How do stocks and bonds play into the future of the organization? 3- What is the future value of money and what is it used for? 4- What is a...
-
Solve each polynomial inequality in Exercises 142 and graph the solution set on a real number line. Express each solution set in interval notation. -x + 2x 0
-
Pappa's Appliances uses the periodic inventory system. Details regarding the inventory of appliances at January 1, purchases invoices during the year, and the inventory count at December 31 are...
-
Provide names for thesecompounds: CI b) CH;CH,COCH,CH3 a) C-OCH,CH,CH3 cuandoen, c) CH,CH,COCH3 d) CH,CH e) NHCH3 h) CN i)
-
Draw structures for these compounds: (a) Propanoyl chloride (b) N, N-Dimethylacetamide (c) Pentatonic anhydride (d) Sodium p-nitro benzoate (e) Hexanamide (f) Isopropyl acetate (g) Benzyl benzoate...
-
Explain which compound has the higher melting point or boilingpoint: a) Higher mp CH;CH,CH,NH, or CH;CN(CH3)2 b) Higher bp CH,CH,CH,COH or CH,CH,COCH; COH CH c) Higher bp CH, or Higher mp...
-
please help Problem 13-7 (Algo) Prepare a Statement of Cash Flows [LO13-1, LO13-2] [The following information applies to the questions displayed below.] Comparative financial statements for Weaver...
-
A firm has 1000 shareholders, each of whom own $59 in shares. The firm uses $28000 to repurchase shares. What percentage of the firm did each of the remaining shareholders own before the repurchase,...
-
Vancouver Bank agrees to lend $ 180,000 to Surrey Corp. on November 1, 2020 and the company signs a six-month, 6% note maturing on May 1, 2021. Surrey Corp. follows IFRS and has a December 31 fiscal...

Study smarter with the SolutionInn App


