Draw the condensation product obtained when the following compound is heated in the presence of aqueous sodium
Question:
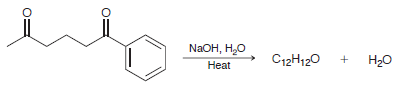
Transcribed Image Text:
NAOH, H,O Нао C12H120 Нeat
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
lilo...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the condensation product obtained when each of the following compounds is heated in the presence of aqueous sodium hydroxide. (a) (b) (c) (d) (e) (f) H.
-
Draw the structure of the product obtained when the following disaccharide is treated with NaBH 4 in methanol. CH- CH- - -
-
Only a substitution product is obtained when the following compound is treated with sodium methoxide: Explain why an elimination product is not obtained. CH3 Br CH3
-
Parker Associates purchased a patent in 2018 for $200,000. The patent will be amortized over 20 years. How would Parker adjust for the annual amortization for the patent on the balance sheet? Credit...
-
What are the six categories of criminal law violations? Describe each, and rank the categories in terms of seriousness.
-
Factor completely. 9k 2 - 12k + 4
-
What are the different elements of an IMC program?
-
The comparative balance sheets for Goltra Company show these changes in noncash current asset accounts: accounts receivable decrease $80,000, prepaid expenses increase $28,000, and inventories...
-
List 3 Financial statements that business entities use on a regular basis. For each statement, explain at least 2 advantages and at least 2 disadvantages of using the statement.
-
Write a program to continuously read input values from the user. The program should terminate if exactly three String values have been inputted. Display the count of integer values and float values...
-
A(n) _____ sample is obtained by dividing the population into groups and selecting all individuals from within a random sample of the groups?
-
A(n) _____ sample is obtained by dividing the population into homogeneous groups and randomly selecting individuals from each group?
-
A bridge is built in the shape of a semielliptical arch. The bridge has a span of 120 feet and a maximum height of 25 feet. Choose a suitable rectangular coordinate system and find the height of the...
-
1. Identify an industry that competes internationally (i.e., fast food, clothing, sportswear, automotive, etc). All your companies must be from ONE Industry (you cannot discuss Taco Bell and Nike)....
-
A research article on " Leadership in Project Management: Cultivating Strong Employee-Employer Bonds" shows major findings on why big companies fail in leadership skill practice. How they can...
-
Discuss and Identify the current types of stock, such as common or preferred stock, currently issued, and outstanding. Include a narrative description along with the values and number of shares found...
-
The organization we intend to study is Local Point, a student cafeteria run by UW Housing & Food Services. Our team would like to figure out how to utilize modern technology and rational...
-
Briefly summarize the Coase Theorem (include the 3 key conditions). List the major types of approaches government typically takes to deal with negative externalities. Suppose the demand for...
-
Determine whether each statement is true or false. If it is false, explain why. The union of the solution sets of x + 1 = 6 x + 1 < 6, and x + 1 > 6 is (-, ).
-
The diagram shows the two forces acting on a small object. Which of the following is the resultant force on the object? A. 8 N downwards B. 8 N upwards C. 2 N downwards D. 2 N upwards 3 N 5 N
-
Show how 2-cyclopentyl-N,N-dimethylethanamine could be synthesized from each of the following starting materials: (a) (b) CH2 CN O- CH2 CHO (two ways)
-
Illustrate the Brgnsted basicity of Mescalino n.q by giving the structures of their conjugate acids,
-
Give the principal organic product(s) expected when N-methylaniline reacts with each of the following reagents. (a) Br2 (b) Benzoyl chloride (c) Benzyl chloride (excess), then dilute -OH (d)...
-
A firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8....
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...

Study smarter with the SolutionInn App


