Draw the polymer that would be generated from the acid-catalyzed reaction between oxalic acid and resorcinol. HO
Question:
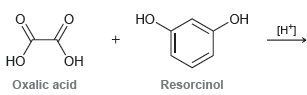
Transcribed Image Text:
HO [H*) НО Он Но Oxalic acid Resorcinol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
HO OH ox...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Heart pacemakers are often powered by lithium-silver chromate "button" batteries. The overall cell reaction is 2 Li(s) + Ag2CrO4(s) Li2CrO4(s) + 2 Ag(s) (a) Lithium metal is the reactant at one of...
-
Draw the polymer that results from the polymerization of vinyl chloride. 3: C: H. CI
-
Identify the condensation polymer that would be produced when phosgene is treated with 1,4 butanediol.
-
23 On January 1, 2021, a company had an existing deferred tax liability attributable to a temporary difference of $20,000. The tax rate at this time was 30%. During 2021, a new law was passed that...
-
Selected cost data for Classic Print Co. are as follows: Estimated manufacturing overhead cost for the year ................... $ 125,000 Estimated direct labor cost for the year...
-
What is a trajectory? What kind of information do you need to predict the trajectory of a particle?
-
Temperature A meteorologist estimates that the median daily high temperature for the month of July in Pittsburgh is 83 Fahrenheit. The high temperatures (in degrees Fahrenheit) for 15 randomly...
-
Ekman Company issued $1,000,000, 10-year bonds and agreed to make annual sinking fund deposits of $78,000. The deposits are made at the end of each year into an account paying 5% annual interest....
-
Hello experts, I have posted this question many times in chegg, but every time people answering this question to use ai tool like chatgpt, please dont use gpt and other ai tool. Question: Analyze the...
-
The executive search being conducted for Western Bank by Headhunters Inc. may finally be bearing fruit. The position to be filled is a key oneVice President for Information Processingbecause this...
-
Draw the mechanism of formation of PET in acidic conditions. It might be helpful to first review the mechanism for the Fischer esterification process.
-
Kevlar is a condensation polymer used in the manufacture of bulletproof vests. Identify the monomers required for the preparation of Kevlar. Kevlar
-
Raka Ltd. provides supplementary post-employment benefits to its retirees. The benefits include dental, vision, and prescription medication. At the beginning of 20X3, Raka had an accumulated OCI loss...
-
Activity 1.4: When Less Becomes More For this activity, refer to the images shown. This is an activity which was performed for you if you do not have available two identical mirrors at home. But if...
-
! Required information [The following information applies to the questions displayed below.] Aces Incorporated, a manufacturer of tennis rackets, began operations this year. The company produced...
-
During the early part of winter, one morning, two hunters decided to go quail hunting on a property where the owner had given them permission to hunt. A nearby forest ranger saw the hunters and...
-
Required information [The following information applies to the questions displayed below.] Trini Company set the following standard costs per unit for its single product. Direct materials (30 pounds...
-
A horticulturist knows that the weights of honeybees that have previously visited her orchard are normally distributed with a mean of 0.87 grams, and a population standard deviation of 0.15 grams....
-
Evaluate the following integrals. A sketch of the region of integration may be useful. ISS 0 yze* dx dz dy
-
Refer to Exercise 8.S.I. Construct a scatterplot of the data. Does the appearance of the scatterplot indicate that the pairing was effective? Explain. Exercise 8.S.I. A volunteer working at an animal...
-
Two structures of the sugar fructose are shown next. The cyclic structure predominates in aqueous solution. (a) Number the carbon atoms in the cyclic structure. What is the functional group at C2 in...
-
Hydration of alkynes (via oxymercuration) gives good yields of single compounds only with symmetrical or terminal alkynes. Show what the products would be from hydration of each compound. (a)...
-
Which of the following compounds would give a positive Tollens test? (Remember that the Tollens test involves mild basic aqueous conditions.) (a) CH3CH2CH2COCH3 (b) CH3CH2CH2CH2CHO (c)...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


