Draw the product formed when each of the following compounds is treated with NaNO 2 and HCl:
Question:
(a)
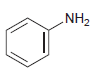
(b)
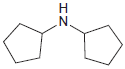
Transcribed Image Text:
NH2 N.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
a b...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the major product formed when each of the following compounds is treated with Et2CuLi followed by mild acid. (a) (b) (c) CN
-
Draw the enolate that is formed when each of the following compounds is treated with LDA. (a) (b) (c) (d)
-
Give the product expected when each of the following compounds is treated with acid. CO.H CO,H + heat)
-
Given the following information, prepare a production report with materials added at the beginning and ending work in process inventory being 20% complete with regard to conversion costs. Costs to...
-
A paper manufacturer buys a machine for $120,000. It depreciates at a rate of 30% per year. (In other words, at the end of each year the depreciated value is 70% of what it was at the beginning of...
-
Within an orthodontic practice that I work in, insufficient patient care and poor time management are the most significant issues in the office. Beginning with the receptionists, scheduling...
-
P 16-9 Recording new partner investmentVarious situations Three partners, Pat, Mic, and Hay, have capital balances and profit-sharing ratios at December 31, 2016, as follows: Pat $144,000 profit...
-
The owner of Genuine Subs, Inc., hopes to expand the present operation by adding one new outlet. She has studied three locations. Each would have the same labor and materials costs (food, serving...
-
12 -60 25 K Prex12 of 14 e n
-
The probability that a college bookstore sells 0, 1, 2, or 3 statistical calculators on any given day is 4/9, 2/9, 2/9, and 1/9, respectively. Construct a probability distribution for the data and...
-
Choose one of the following products and explain how it blurs the distinction between goods and services. a. Knee replacement surgery b. Dinner at a popular restaurant c. Purchase and installation of...
-
Consider the structure of the azo dye called alizarine yellow R (below). Show the reagents you would use to prepare this compound via an azo coupling process. .N. N' O2N
-
The unadjusted trial balance for Taylor Electronics Company follows: Requirements 1. Journalize the adjusting entries using the following data: a. Interest revenue accrued $ 200. b. Salaries...
-
Caching. CPUs. Hard disk drives. Parallelism. Pipelining. RAM. Registers. Solid-state drives. Dinkleberg 1. (3 points) You know that neighbor who always one-ups you? Apparently, he just bought a...
-
Information for Question 1 and 2: The National Health Interview Survey is an annual national health survey of people living in about 35,000 randomly selected households in the United States. On the...
-
Q1. There is trampoline on the ground, and connected to spring of spring constant, k, on the bottom. You are holding a child of mass, M on a H tall building top (H is measured from the trampoline to...
-
SPSS for intermediate statistics extra problems solutions d. Approximately how much variance in consumers' tendency to purchase the product could be predicted from these variables? If you owned the...
-
A finance company uses the discount method of calculating interest. The loan principal is $5,000, the interest rate is 10%, and repayment is expected in two years. You will receive 4196.25 X from the...
-
The figure shows the cost of mailing a first-class letter, f(x), as a function of its weight, x, in ounces, for a recent year. Use the graph to solve Exercises 111114. What is the cost of mailing a...
-
Cassandra Casey operates the Futuristic Antique Store. She maintains subsidiary ledgers for accounts payable and accounts receivable. She presents you with the following information for October 2019:...
-
Sulfur dioxide has a dipole moment of 1.60 D. Carbon dioxide has a dipole moment of zero, even though C-O bonds are more polar than S-O bonds. Explain this apparent contradiction.
-
For each of the following compounds, 1. Draw the Lewis structure. 2. Show how the bond dipole moments (and those of any nonbonding pairs of electrons) contribute to the molecular dipole moment. 3....
-
Diethyl ether and butan-1-ol are isomers, and they have similar solubilities in water. Their boiling points are very different, however. Explain why these two compounds have similar solubility...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


