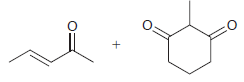
Draw the product of the Robinson annulation reaction that occurs when the following compounds are treated with
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
NaOH Micha...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following reaction illustrates the Robinson annulation reaction (Section 19.7A). Provide a mechanism. base
-
Identify what reagents you would use to make the following compound with a Robinson annulation reaction.
-
Show the resonance forms for the enolate ions that result when the following compounds are treated with a strong base. (a) Ethyl acetoacetate (b) Pentane-2,4-dione (c) Ethyl a-cyanoacetate (d)...
-
Using the case study, characterize Amazons approach to marketing communications.
-
For the one-dimensional fluid-flow problem in Figure P14-6, determine the velocities and volumetric flow rates at nodes 2 and 3. Let Kxx = 10-1 in./s? Figure P14-6 4 in./s 1 A, 2 in.? 2. Az = 1 in.?...
-
Suppose that the given expressions are denominators of rational expressions. Find the least common denominator (LCD) for each group of denominators. 2y + 8, y + 4
-
An increase in sales _________ the P/E ratio.
-
A work cell at Chris Ellis Commercial Laundry has a workstation with two machines, and each unit produced at the station needs to be processed by both of the machines. (The same unit cannot be worked...
-
Instructions On January 1, 2019, Loud Company enters into a 2-year contract with a customer for an unlimited talk and 5 GB data wireless plan for $64.00 per month. The contract includes a smartphone...
-
Please solve this problem using C language Hacker Industries has a number of employees. The company assigns each employee a numeric evaluation score and stores these scores in a list. A manager is...
-
Research supports the argument that the way we pay for a product changes the way we perceive it. More specifically,credit cards prime people to focus less on the costs of the item and more on the...
-
Draw a plausible mechanism for the following transformation. NO2 NO2 NaOH, H,0 eat
-
Do you think that a general increase in the extent of separation between the ownership and management of organisations over time has led to a greater or a lesser amount of accounting regulation? Why?
-
Solve the following linear system by Gaussian elimination with back-substitution without introducing fractions in your row-reduction. If there is no solution, explain why. -3x+8y + 82 = -8 -2x+ y -...
-
Introduction Some predictions are a slam dunk. Retail will continue to be driven by technology. Science fiction is coming to life in the form of robotics and virtual reality. And the Internet will...
-
Oswego Clay Pipe Company provides services of $ 5 0 , 0 0 0 to Southeast Water District # 4 5 on April 1 2 of the current year with terms 1 / 1 5 , n / 6 0 . What would Oswego record on April 1 2 ?...
-
Assume the following excerpts from a company's balance sheet: Property, plant, and equipment Beginning Balance $3,500,000 Ending Balance $3,700,000 $1,100,000 $800,000 Long-term investments During...
-
On January 1, 2021, Bonita Corp. had472,000shares of common stock outstanding. During 2021, it had the following transactions that affected the Common Stock account. February 1 Issued 125,000shares...
-
Let A = {1, 2, 3, 4, 5, 6}, B = {1, 3, 5}, C = {1, 6}, and D = {4}. Find each set. A B
-
Determine the center and radius of each circle. Sketch each circle. 4x 2 + 4y 2 9 = 16y
-
Draw the structure(s) of (a) an achiral ketopentose C5H10O5 (b) -D-idofuranose
-
Specify the relationship(s) of the compounds in each of the following sets. Choose among the following terms: identical compounds, epimers, anomers, enantiomers, diastereomers, constitutional...
-
Tell whether each structure or term is a correct description of the L-sorbose structure shown here or a form with which it is in equilibrium. (b) a ketohexose (d) A ketohexose (f) An aldohexose CH2OH...
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


