Each compound below exhibits one lone pair. In each case, identify the type of atomic orbital in
Question:
a.
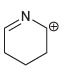
b.
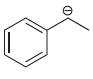
c.
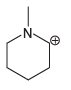
Transcribed Image Text:
.N.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
a An sp 2 hy...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine whether each compound below exhibits a molecular dipole moment: a) CH 4 b) NH 3 c) H 2 O d) CO 2 e) CCl 4 f) CH 2 Br 2
-
For each compound below, identify all lone pairs and indicate whether each lone pair is localized or delocalized. Then, use that information to determine the hybridization state and geometry for each...
-
The compound 1,1,1-trifl uoroacetylacetone (tfa) is a bidentate ligand: It forms a tetrahedral complex with Be2+ and a square planar complex with Cu2+. Draw structures of these complex ions and...
-
In Exercises find the second derivative of the function. f(x) = x + 3x-3
-
On August 1, Clayton Co. issued 1,300,000 of 20 year, 9% bonds, dated August 1, for $1,225,000. Interest is payable semiannually on February 1 and August 1. Present the entries to record the...
-
Evaluate the integral. S (x + 2y) dy
-
Warehouse expenses is an example of (a) Factory overhead (b) Administrative overhead (c) Selling overhead (d) Distribution overhead
-
The following activities take place at Lohman CPAs during a recurring external audit of Reliance Corp. Classify the activities as value-added or non-value-added from the perspective of Reliance Corp....
-
Determining Retained Earnings and Net Income Using the Balance Sheet The following information is reported for Kinney Corporation at the end of 2018. Accounts Receivable $23,000 Retained Earnings $?...
-
A survey conducted by the Northwestern University Lindquist-Endicott Report asked 320 companies about the procedures they use in hiring. Only 54% of the responding companies review the applicants...
-
Learning to extract structural information from molecular formulas: a) Write out the molecular formula for each of the following compounds: Compare the molecular formulas for the above compounds and...
-
Draw all significant resonance structures for each of the following compounds: a. b. c. N' z-
-
Charles Austin of the controllers office of Thompson Corporation was given the assignment of determining the basic and diluted earnings per share values for the year ending December 31, 2026. Austin...
-
What could a team leader do to determine whether individuals or teams require extra support?
-
Write a MATLAB script to visualize a parametric surface representing a torus ( doughnut shape ) ?in 3 D space. The parametric equations for a torus with major radius R and minor radius r are given...
-
Read the synopsis just above or next to the video clip, then view the clip in its entirety. here is the link https://broadwayeconomics.com/gaston/ https://broadwayeconomics.com/gaston/. (In some...
-
On your 23rd birthday you decide to invest $4,500 (10% of your annual salary) in a mutual fund earning 7% per year. You will continue to make annual deposits equal to 10% of your annual salary until...
-
The graph of a function f is given. Sketch the graphs of the following transformations of f. y 5 -4 -2 2 4 6 5 00 8 10 10 x
-
Solve by any method. Assume that a and b represent nonzero constants. ax + by = 2 - ax + 2by = 1
-
Find a polar equation for the curve represented by the given Cartesian equation. 4y 2 = x
-
Using the method of Section 9.8A, determine the number of expected signals for the following compounds. (a) (b) (c) CH3
-
Compound S (C8H16) reacts with one mole of bromine to form a compound with molecular formula C8H16Br2. The broadband proton-decoupled 13C spectrum of S is given in Fig. 9.49. Propose a structure for...
-
A compound with molecular formula C4H8O has a strong IR absorption at 1730 cm-1. Its mass spectrum includes key peaks at m/z 44 (the base peak) and m/z 29. Propose a structure for the compound and...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


