Draw all significant resonance structures for each of the following compounds: a. b. c. N' z-
Question:
a.
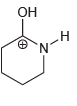
b.
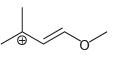
c.
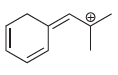
Transcribed Image Text:
Он н N' z-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a b ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw all significant resonance structures for each of the following compounds: Testosterone Estradiol (Female sex hormone) (Male sex hormone)
-
Draw contributing resonance structures for each of the following species, and rank the structures in order of decreasing contribution to the hybrid: a. b. c. d. e. f. CH3C-CH CHCH3 CH3 0 CH3COCH3 +OH...
-
Draw resonance structures for each of the following radicals: (a) (b) (c) (d)
-
In Exercises evaluate the second derivative of the function at the given point. Use a computer algebra system to verify your result. (x) = cos x, (0, 1)
-
On April 31, 2016, Elkhorn Associates borrowed $10 million cash from Colonial Bank and issued a 5-month, noninterest-bearing note, priced to yield an effective interest rate of 10%. The stated...
-
Find the volume of the solid of intersection of the three cylinders x + z = 1, y + z = 1, and x + y = 1 (see figure). 3 3 2 -3+ 3 -3 3 Z -3 3 -
-
Which of the following is not a selling overhead? (a) Royalty on sales (b) Distribution of samples (c) Legal cost for debt realization (d) Insurance to cover sold goods while in transit
-
Here are two useful rules of thumb. The Rule of 72 says that with discrete compounding the time it takes for an investment to double in value is roughly 72/interest rate (in percent). The Rule of 69...
-
Entries for Sale of Fixed Asset Equipment acquired on January 8 at a cost of $99,310 has an estimated useful life of 12 years, has an estimated residual value of $8,950, and is depreciated by the...
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Each compound below exhibits one lone pair. In each case, identify the type of atomic orbital in which the lone pair is contained. a. b. c. .N.
-
Write a condensed structural formula for each of the following compounds: a. b. c. HO
-
Ramirez Company has the following data for the weekly payroll ending January 31. Employees are paid 1 times the regular hourly rate for all hours worked in excess of 40 hours per week. The 7.65% FICA...
-
Two roommates (Jen and Kate) can choose whether to clean their apartment (C) or leave it dirty (D). Jen's cost of cleaning is c, but Kate doesn't mind cleaning and has no cost. [Recall that their...
-
Designation Mass per Depth Width Thickness metre of of section section of of web flange Root Depth radius between Ratios for local buckling Second moment of area Radius of gyration fillets | i Flange...
-
Discuss the attributes that make an effective leader. What tenets should a leader practice? How does leadership directly impact effective public management? In your own experience, what has led you...
-
Given the following examples identify whether it describes a positive externality, negative externality, or neither. Example 1: Johanna is graduating from college this weekend. Like her, individuals...
-
32) Suppose Joaquin grows at an average rate of 0.5in/year for 3 years, then 1.25 inches/year for 4 years, then 0.75 inches/year for 4 years, then 0.4in/year for 5 years. In that time span, how much...
-
Solve by any method. Assume that a and b represent nonzero constants. 2ax - y = 3 y = 5ax
-
Find the velocity, acceleration, and speed of a particle with the given position function. r(t) = (t 2 , sin t - t cos t, cos t + t sin t), t > 0
-
In the mass spectrum of 2, 6-dimethyl-4-heptanol there are prominent peaks at m/z 87, 111, and 126. Propose reasonable structures for these fragment ions.
-
In the mass spectrum of 4-methyl-2-pentanone a McLafferty rearrangement and two other major fragmentation pathways occur. Propose reasonable structures for these fragment ions and specify the m/z...
-
What are the masses and structures of the ions produced in the following cleavage pathways? (a) A-cleavage of 2-methyl-3-hexanone (two pathways) (b) Dehydration of cyclopentanol (c) McLafferty...
-
You are evaluating a new project for the firm you work for, a publicly listed firm. The firm typically finances new projects using the same mix of financing as in its capital structure, but this...
-
state, "The subscription price during a rights offering is normally r; lower ; lower r; higher er; higher than the rights-on price and
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...

Study smarter with the SolutionInn App


