Each pair of compounds below will undergo an acidbase reaction. In each case, identify the acid, identify
Question:
(a)
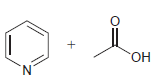
(b)
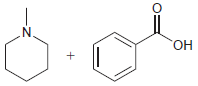
Transcribed Image Text:
ОН `N' N. HO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a b N...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each pair of compounds below, identify the stronger base: (a) (b) (c) (d) (e) (f) -
-
For each pair of compounds, identify the stronger base. (a) (b) (c) vs. N. N. z. Vs. %3D N. N.
-
For each pair of compounds below, identify the more acidic compound: (a) (b) (c) (d) (e) (f) (b) C (d) H. -S -3- (f) X H' `H.
-
Suppose that you are holding your toy submarine under the water. You release it and it begins to ascend. The graph models the depth of the submarine as a function of time. What is the domain and...
-
Rite Aid pharmacy in Big Rapids, Michigan is using simple exponential smoothing to predict monthly birthday card sales. At the end of October 2004, the pharmacy's forecast for December 2004 sales was...
-
The logistic growth function models the percentage, P(x), of Americans who are x years old with some coronary heart disease. Use the function to solve Exercises 4346. At what age is the percentage of...
-
4. Fox, Gre, and How are partners with average capital balances during 2016 of $120,000, $60,000, and $40,000, respectively. Partners receive 10 percent interest on their average capital balances....
-
Bergamo Bays computer system generated the following trial balance on December 31, 2015. The companys manager knows something is wrong with the trial balance it does not show any balance for work in...
-
Thornton Industries began construction of a warehouse on July 1, 2021. The project was completed on March 31, 2022. No new loans were required to fund construction. Thornton does have the following...
-
You need to calculate the estimated future availability of a cloud-based IT resource. You determine that only one possible event can occur for the cloud-based IT resource to become unavailable. As a...
-
Draw all tertiary amines with molecular formula C 5 H 13 N, and provide a name for each isomer. Are any of these compounds chiral?
-
1. Assess Prairie Home Appliance Service. Is the company performing well? Are they fulfilling their mission? 2. What do you think of Becky Freeman? Evaluate her qualifications and performance to...
-
What do you consider to be the key challenges in coding data from an open-ended interview?
-
Solve the Differential equation. xydx+dy=0
-
What is Hue and saturation?
-
Explain traversing on the following parcel.Provide one numerical example.
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Analyze the impact of sustainable construction on biodiversity and ecosystem services. How can construction practices be adapted to minimize impacts on local ecosystems and enhance biodiversity?
-
The wage gap is used to compare the status of womens earnings relative to mens. The wage gap is expressed as a percent and is calculated by dividing the median, or middlemost, annual earnings for...
-
On October 1, 2021, Adoll Company acquired 2,600 shares of its $1 par value stock for $38 per share and held these shares in treasury. On March 1, 2023, Adoll resold all the treasury shares for $34...
-
Draw a Lewis structure for each compound. Include all nonbonding pairs of electrons. (a) CH3COCH2CHCHCOOH (b) NCCH2COCH2CHO (c) CH2CHCH(OH)CH2CO2H (d) CH2CHC(CH3)CHCOOCH3
-
Draw a line-angle formula for each compound in Problem 1-26. In problem (a) CH3COCH2CHCHCOOH (b) NCCH2COCH2CHO (c) CH2CHCH(OH)CH2CO2H (d) CH2CHC(CH3)CHCOOCH3
-
Draw Lewis structures for (a) Two compounds of formula C4H10 (b) Two compounds of formula C2H6O (c) Two compounds of formula C2H7N (d) Three compounds of formula C2H7NO (e) Three compounds of formula...
-
Summarize in your own words Sharps, Treynors, and Jensens Measures for assessing portfolio performance with respect to risk. Assess the portfolio performance of mutual fund VDIGX taking into...
-
Question 1 Slat and Company have recently set up a business which will manufacture and sell a furniture component, the F12 On the 19 August 2021, the company issued 85,000 of share capital for cash....
-
The following is Addison Corporations contribution format income statements for last month. The company has no beginning or ending inventories. A total of 10,000 units were produced and sold last...

Study smarter with the SolutionInn App


