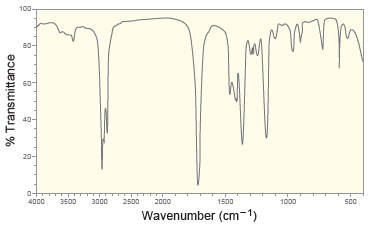
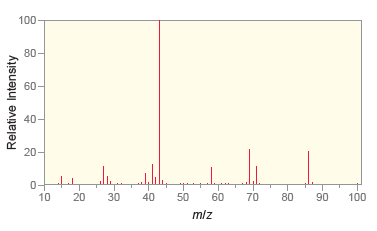
Following are the IR spectrum and mass spectrum of an unknown compound. Propose at least two possible
Question:
Transcribed Image Text:
100- 80- 40- 20- 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm-1) % Transmittance 100 80- 60- 40- 20- 10 20 30 40 50 60 70 80 90 100 mlz Relative Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The IR spetrum indicates that the compound is a ketone T...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Propose at least two different structures for a compound with six carbon atoms that exhibits the following features: a. All six carbon atoms are sp 2 hybridized. b. Only one carbon atom is sp...
-
Propose at least two strategies to avoid assumptions in a multiyear plan. Justify your response. Recommend at least two best practices for analyzing multiyear financial statements. Justify your...
-
An unknown compound gives a mass spectrum with a weak molecular ion at m/z 113 and a prominent ion at m z 68. Its NMR and IR spectra are shown here. Determine the structure, and show how it is...
-
In Exercises 7192, find and simplify the difference quotient f(x +h)-f(x) h -, h = 0
-
Define the following: 1. Select database management system (Oracle, SQL Server, MYSQL, etc) and identify the data types and sizes for all attributes. 2. Make sure all relationships have been...
-
For each of the following enthymematic arguments: a. Formulate the plausible premise or conclusion, if any, that is missing but understood. b. Write the argument in standard form, including the...
-
4. Suppose you observe the following 1-year implied forward rates: 0.050000 (1- year), 0.034061 (2-year), 0.036012 (3-year), 0.024092 (4-year), 0.001470 (5-year). For each maturity year compute the...
-
Siberian Ski Company recently expanded its manufacturing capacity, which will allow it to produce up to 15,000 pairs of cross-country skis of the mountaineering model or the touring model. The sales...
-
Current Position Analysis The following data were taken from the balance sheet of Nilo Company at the end of two recent fiscal years: Current Year Previous Year Current assets: Cash $279,300 $206,400...
-
Fortune magazine's list of the world's most admired companies for 2014 is provided the data contained in the WEB file named Admired Companies (Fortune, March 17, 2014). The data in the column...
-
Calculate the HDI for each molecular formula. a) C 4 H 6 b) C 5 H 8 c) C 40 H 78 d) C 72 H 74 e) C 6 H 6 O 2 f) C 7 H 9 NO 2 g) C 8 H 10 N 2 O h) C 5 H 7 Cl 3 i) C 6 H 5 Br j) C 6 H 12 O 6
-
Following are the IR spectrum and mass spectrum of an unknown compound. Propose at least two possible structures for the unknown compound. 100 80 20 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber...
-
Which of the following is not a method on Session interface? (a) save() (b) remove() (c) saveorupdate() (d) load()
-
Lifestyle is how one enacts the self-concept. The way they would enact it is through buying luxury items which is the most premium iPhone. The latent reasons why people want an iPhone 15 all have to...
-
Make a Tows Matrix that assess the strengths, weakness, opportunities, and threats for Dannon based on the case study For typical corporate strategies under purpose of communication. Strengths 1) 2)...
-
Now that you've watched the lectures, The Abilene Paradox movie, and the Challenger Disaster Video, I'd like you to think for a moment about when you may have observed the Abilene Paradox or...
-
Ensuring that the projectadheres to the selected quality standard . Often, ensuring that the project work is done 'correctly' is as important as ensuring that the end result fulfills the project's...
-
Think about some career planning and development issues; for example, mergers and reorganization uncertainty, lack of upward mobility, getting managers to understand your career potential, and...
-
Use transformations of the graph of either f(x) = 1/x or h(x) = 1/x 2 to sketch a graph of y= g(x) by hand. Show all asymptotes. Write g(x) in terms of either f(x) or h(x). g(x) = 2 (x - 1)
-
Planning: Creating an Audience Profile; Collaboration: Team Projects. Compare the Facebook pages of three companies in the same industry. Analyze the content on all available tabs. What can you...
-
Into the same funnel is poured carefully 50 mL of hexane (density = 0.660 g/ml) so that the other two layers are not disturbed. The hexane forms a third layer. The funnel is stoppered and the mixture...
-
Give a general balanced reaction for The complete combustion of a cycloalkane containing one ring formula CnH2n.
-
Carv and Di Oxhide drive their family car about12,000 miles per year. Their car gets about 25 miles per gallon of gasoline. "What is the carbon footprint" (pounds of CO, released into the atmosphere)...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


