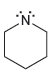
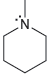
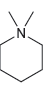
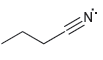
For each of the compounds below determine whether any of the nitrogen atoms bear a formal charge:
Question:
a.
b.
c.
d.
Transcribed Image Text:
N.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a b No...View the full answer

Answered By

Stacy kosgei
I offer quality, original and timely services; Highly credible and void of plagiarism. Your success is my pleasure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the compounds below determine whether any of the oxygen atoms bear a formal charge: a. b. c. d. ::
-
For each of the compounds below, locate the pattern we just learned (lone pair next to a Ï bond) and draw the appropriate resonance structure: a. b. c. d. e. f. g. h. NH2
-
For each of the compounds below, locate the lone pair adjacent to a positive charge and draw the resonance structure: a. b. c. N.
-
Refer to E 29 and respond to the following requirements. Data in E 2-9 Prepare the necessary adjusting entries on December 31, 2024, for the Microchip Company for each of the following situations....
-
On January 1, 2010, ABC Co. had inventory of 200 units @ 18.00 a unit. It purchased 400 more units @ $20.00 a piece on Feb. 19th. On May 12th, it bought 300 more units @ $22.00 each. On Oct. 3rd, it...
-
Use a computer algebra system to evaluate the iterated integral. *2 (1+cos 0 ST. 0 6r cos 0 dr de
-
15. Under what circumstances will a proprietary fund be required to report segment information?
-
Gibbs Corporation produces industrial robots for high-precision manufacturing. The following information is given for Gibbs Corporation. The company has a desired ROI of 20%. It has invested assets...
-
Problem 15-16 Common-Size Financial Statements (LO15-1] You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a...
-
An optical storage device uses and error recovery Procedure that requires an immediate satisfactory readback of any written data. If the readback is not successful after three writing operations,...
-
Atenolol and enalapril are drugs used in the treatment of heart disease. Both of these drugs lower blood pressure (albeit in different ways) and reduce the risk of heart attack. Using the following...
-
Draw all lone pairs on each of the oxygen atoms in the compounds below. Before doing this, review in the following table, and then come back to these problems. Try to identify all lone pairs without...
-
Ciaras Cookie Company provided the following accounts from its year- end trial balance. Ciara's Cookie Company Unadjusted Trial Balance (Selected Accounts) For the CurrentYear Ended Debit Credit S...
-
The Role of Leadership in Shaping Organizational Culture Recent research stated that [c]ompanies with an established organizational culture that includes strong capabilities for change, commitment to...
-
Unscheduled absences by clerical and production workers are an important cost in many companies. Reducing the rate of absenteeism is, therefore, an important goal for a company's human relations...
-
Many of the largest tech firms, including Google, Apple, Amazon, and Microsoft, have spent hundreds of millions of dollars to improve their information technology infrastructure. Now, these companies...
-
In the business sense, a product refers to a commodity available for purchase, encompassing both services and tangible or intangible items. It may exist in physical, virtual, or cyber forms. Every...
-
Data Exploration and Multiple Linear Regression (MLR) using SAS. The "College" data set contains the statistics for many US Colleges from 1995 issue of US News and World Report. It has 777...
-
Solve each system using the substitution method. If a system is inconsistent or has dependent equations, say so. 1 2 x+ - 3x 1 y = = 3 + y = 0
-
Where are the olfactory sensory neurons, and why is that site poorly suited for their job?
-
Starting with an appropriate alkyl halide and base, outline syntheses that would yield each of the following alkenes as the major (or only) product: (a) (b) (c) (d) (e)
-
Predict the more stable alkene of each pair: (a) 2-methyl-2-pentene or 2,3-dimethyl- 2-butene; (b) Cis-3-hexene or trans-3-hexene; (c) 1-hexene or cis-3-hexene; (d) Trans-2- hexene or...
-
Arrange the following alcohols in order of their reactivity toward acid-catalyzed dehydration (with the most reactive first): 1-Pentanol 2-Methyl-2-butanol 3-Methyl-2-butanol
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


