For each of the compounds below determine whether any of the oxygen atoms bear a formal charge:
Question:
a.
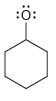
b.
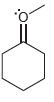
c.
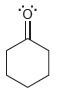
d.
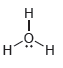
Transcribed Image Text:
:ö:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a b ...View the full answer

Answered By

Amit Choudhary
I'm new in this profession regarding online teaching but previously i used to teach students near my college. I am teaching on online platform since last year and got good support from the students. I'm teaching on platforms like chegg and vedantu and also at my home in free time.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the compounds below determine whether any of the nitrogen atoms bear a formal charge: a. b. c. d. N.
-
For each of the compounds below, locate the pattern we just learned (lone pair next to a Ï bond) and draw the appropriate resonance structure: a. b. c. d. e. f. g. h. NH2
-
For each of the compounds below, locate the lone pair adjacent to a positive charge and draw the resonance structure: a. b. c. N.
-
Which of the units listed in Multiple-Choice Question 9 are appropriate for the biologically equivalent dose that results when a person is exposed to radiation? Data From Question 9 Which of these...
-
On January 1, 20Y1, Martin Manufacturing paid cash for a new piece of manufacturing equipment. The machine cost $40,000 and had an estimated useful life of 5 years with a $5,000 salvage value ....
-
Show that the function is a joint density function and find the required probability. f(x, y) [e-x-y, x 0, y = 0 elsewhere 0, P(0 x 1, x y 1)
-
16. How might the enterprise fund amounts on the proprietary fund statement of net position differ from the amounts reported as business-type activities on the government-wide statement of net...
-
The FASB has been working on a conceptual framework for financial accounting and reporting and has issued seven Statements of Financial Accounting Concepts. These SFAC s are intended to set forth...
-
A company's ledger accounts and their end-of-period balances before closing entries are posted are shown below. What amount will be posted to retained earnings in the process of closing the Income...
-
Dodge City Realty acts as an agent in buying, selling, renting, and managing real estate. The unadjusted trial balance on July 31, 2010, is shown below. The following business transactions were...
-
Atenolol and enalapril are drugs used in the treatment of heart disease. Both of these drugs lower blood pressure (albeit in different ways) and reduce the risk of heart attack. Using the following...
-
Draw all lone pairs on each of the oxygen atoms in the compounds below. Before doing this, review in the following table, and then come back to these problems. Try to identify all lone pairs without...
-
Which federal statute requires that credit reports can only be obtained for legitimate business needs? a. Title 15 U.S. Code 1692. b. Final Rule45 CFT Parts 160 and 165. c. Title 15 U.S. Code,...
-
Based on the reading,How to make sure your next product or service launch drives growth (click the underlined link),what stands out to you as the most important factor in a differentiated launch...
-
OM in the News has previously looked at the Waffle House Index, used to measure the damage from hurricanes. The index made the news again for Hurricane Ian. According to the Boston Globe, 40 Waffle...
-
Mixture of persuasive and negative formal I am Elizabeth grinderFirst part email Next part setting up the meeting Final part memo The reader is Robert * do not come off accusatory*** Project TWO:...
-
Anyone who has sampled today's social media offerings has probably experienced this situation: You find a few fascinating blogs, a few interesting people to follow on Twitter, a couple of podcast...
-
a. Begin with a converging lens of focal length f. Place an illuminated object a distance p, in front of the lens. For all positive values of p;: 1. calculate and sketch a graph of the location of...
-
A truck radiator holds 36 L of fluid. How much pure antifreeze must be added to a mixture that is 4% antifreeze to fill the radiator with a mixture that is 20% antifreeze?
-
Dan and Diana file a joint return. Dan earned $31,000 during the year before losing his job. Diana received Social Security benefits of $5,000. a. Determine the taxable portion of the Social Security...
-
Give the products that would be formed when each of the following alcohols is subjected to acid-catalyzed dehydration. If more than one product would be formed, designate the alkene that would be the...
-
1-Bromobicyclo [2.2.1] heptane does not undergo elimination (below) when heated with a base. Explain this failure to react. (Construction of molecular models may help.) Br
-
When the deuterium-labeled compound shown at right is subjected to dehydrohalogenation using sodium ethoxide in ethanol, the only alkene product is 3-methylcyclohexene. (The product contains no...
-
Domino is 4 0 years old and is married out of community of property with the exclusion of the accrual system to Dolly ( 3 5 ) . They have one child, Domonique, who is 1 1 years old. Domino resigned...
-
YOU ARE CREATING AN INVESTMENT POLICY STATEMENT FOR JANE DOE General: 60 years old, 3 grown children that are living on their own and supporting themselves. She is in a very low tax rate so we don't...
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...

Study smarter with the SolutionInn App


