For each of the following molecules, determine the number of carbon atoms present, and then determine the
Question:
a.
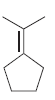
b.
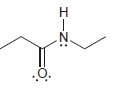
c.
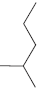
d.
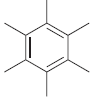
e.
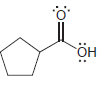
f.
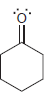
g.
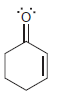
h.
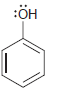
i.
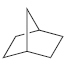
j.
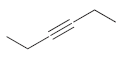
k.
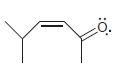
l.
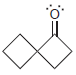
Transcribed Image Text:
-z:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a b c d e f g ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following molecules that contain polar covalent bonds, indicate the positive and negative ends of the dipole, using the symbol v. Refer to Table 1.2 as needed. (a) HCI (c) HI (e) HOCI...
-
For each of the following molecules, state the bond angle (or bond angles, as appropriate) that you would expect to see on the central atom based on the simple VSEPR model. Would you expect the...
-
For each of the following molecules, state the bond angle (or bond angles, as appropriate) that you would expect to see on the central atom based on the simple VSEPR model. Would you expect the...
-
Debbie plans to buy a house for cash instead of paying a mortgage. She is willing to set aside $12 000 at the end of each year for 15 years. She puts her savings in a Tax Free Savings Account (TFSA)...
-
Define the velocity of money. What determines velocity? How are changes in velocity related to inflation/deflation?
-
What is the explanation for why the average forecasting errors were higher for the other time series forecasting methods than for the supposedly less powerful last-value method?
-
Describe the qualities of a good project metric. AppendixLO1
-
What two important management principles affect the successful operation of a growing organization? Do you think John Moodys difficulties could have been avoided if he understood these principles?...
-
On January 1, 2017, Mace Co. acquired 75% of Lance Co.'s outstanding common stock. On the same date, Lance acquired an 80% interest in Curle Co. Both of these investments were acquired when book...
-
f(x) = 2x + 3 and g(x) = -3x + 2. For the above function, use base point x0 = 1.0 and x = 0.1 to compute f and g. Find (fg) (the change in the product) by computing f(x0 + x)g(x0 + x) - f(x0)g(x0)....
-
Below are mass spectra for four different compounds. Identify whether each of these compounds contains a bromine atom, a chlorine atom, or neither. a. b. c. d. 100- 80- 60- 60- 40 20- 0- 60 70 10 20...
-
In Section 4.2, we learned how to name bicyclic compounds. Using those rules, together with the rules discussed in this section, provide a systematic name for the following bicyclic compound:
-
9. Let S = \($40\), K = \($45\), = 0.30, r = 0.08, and = 0. Compute the value of knockout calls with a barrier of \($60\) and times to expiration of 1 month, 2 months, and so on, up to 1 year. As...
-
A pistoncylinder device contains 0.85 kg of refrigerant-134a at 210 oC. The piston that is free to move has a mass of 12 kg and a diameter of 25 cm. The local atmospheric pressure is 88 kPa. Now,...
-
3.3. Using the BEMT, show the effect of increasing linear twist on the variations in inflow, thrust, induced power, profile power, and lift coefficient across the span of a rotor with four blades of...
-
By uploading this work, I attest that the work contained herein is solely my own, that I only used the given equation sheet as a reference, and that I have not received any information from anyone...
-
Demand for patient surgery at Washington General Hospital has increased steadily in the past few years, as seen in the following table: ...
-
Explain product analysis
-
Find f -1 (x). f(x) = (x + 2), x = -2
-
If the amplifier indicated by the box input impedance of oo, which of the following statements are true ? has an open loop gain as well as Feedback factor (\beta = 1/ R_1\) The feedback is voltage...
-
Identify each pair of relationships among the ? OH groups in glucose (red?blue, red?green, red?black, blue?green, blue?black, green?black) as cis or trans. CH2OH OH OH Glucose OH
-
A Draw 1, 3, 5-trimethy1cyclohexane using a hexagon to represent the ring. How many cisTrans stereoisomers are possible?
-
From the data in Figure and Table, estimate the percentages of molecules that have their substituents in an axial orientation for the following compounds: (a) Isopropylcyclohexane (b)...
-
Docs Auto Body has budgeted the costs of the following repair time and parts activities for 2009: Doc's budgets 6,000 hours of repair time in 2009. A profit margin of $7 per labour hour will be added...
-
QUESTION 28 In a perpetual inventory system, the cost of inventory sold is: Debited to accounts receivable. Debited to cost of goods sold. O Not recorded at the time goods are sold. O Credited to...
-
The following financial statements and additional information are reported. IKIBAN INC. Comparative Balance Sheets June 30, 2019 and 2018 2019 2018 $105,709 69,500 66,800 4,700 246,700 127,eee...

Study smarter with the SolutionInn App


