For each of the following pairs of compounds, identify which compound would react more rapidly in an
Question:
(a)
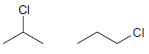
(b)
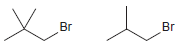
(c)
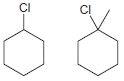
(d)

Transcribed Image Text:
CI .CI Вг Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
a b c ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them: a. b. c. d. e. f. g. h. i. cis-2-butene and trans-2-butene j. CH3CH2CH2OH...
-
For each of the following pairs of compounds, indicate the compound that you would expect to be a more potent inhibitor of dihydrofolate reductase: a. b. NH NH CI of CH3 CH3 NH2 CH3 or H.NNCHCHCHCH,...
-
For each of the following pairs of compounds, determine which compound is more stable (you may find it helpful to draw out the chair conformations): (a) (b) (c) (d) II
-
In Exercises 1114, graph each equation in a rectangular coordinate system. If two functions are indicated, graph both in the same system. Then use your graphs to identify each relations domain and...
-
Identify three reasons why individuals create new business ventures. Next discuss any goals or motives that would drive you to become an entrepreneur. Provide examples for your response. Discuss at...
-
Wen Chu is a senior account manager for a large Canadian bank. The bank is considering the possibility of syndicating a large debenture issue for Wilstar Products Ltd. (WPL), a manufacturer of auto...
-
Williams Company obtains all of the outstanding stock of Jaminson, Inc. In a consolidation pre pared immediately after the takeover, at what value will Jaminsons inventory be consolidated? a. At...
-
Langer Company has three products (A, B, and C) that use common facilities. The relevant data concerning these three products follow. Required If fixed costs allocated to product line C are not...
-
Problem 1 (2 points) : 1.1 Your rich uncle invested $6,000 in an aggressive (i.e., risky) mutual fund 25 years ago. Much to your uncle's chagrin, the value of his investment declined by 18.0% during...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Draw all isomers of C 4 H 9 I, and then arrange them in order of increasing reactivity toward an S N 2 reaction.
-
Consider the following reaction: (a) How would the rate be affected if the concentration of the alkyl halide is doubled? (b) How would the rate be affected if the concentration of sodium cyanide is...
-
According to queuing theory, what is the critical disk utilization percentage?
-
What Do You Know About Amazon Associate Program? What Would You Do To Increase Your Earnings With Amazon Associate Program? Is Affiliate Marketing And Referral Marketing One And The Same? What is...
-
As a leader, what do you think are important elements of a leadership team made up of those senior people that you will surround yourself with? Do you have (or have you had) a mentor? If so, how have...
-
What role do interorganizational relationships and alliances play in achieving strategic goals, and how do organizations manage these relationships to ensure mutual benefit and minimize risks ?
-
How do expatriate managers normally rotate into the operations of a foreign country? How long do they typically stay in the country? What are the disadvantages? How did Shane Tedjarati rotate into...
-
If you are not Asian, do you know someone well who is Asian? In what capacity do you know them (e.g., personal friend, manager, classmate, neighbor, etc.)? Do you know their ethnic origin (e.g.,...
-
Two liters of a 35% sulfuric acid solution need to be diluted to a 20% solution. How many liters of a 12% sulfuric acid solution should be mixed with the 2-liter solution?
-
The first law of thermodynamics is sometimes whimsically stated as, You cant get something for nothing, and the second law as, You cant even break even. Explain how these statements could be...
-
Give structural formulas for the products that you would expect from the following reactions: (a) (b) (c) (d) KMnO, heat B-Pinene H2. Pt Zingiberene HCi Caryophyllene -Selinene (1) BH3 THF (2 equiv.)...
-
Draw the two basic ring systems given in Fig. 23.6 for the 5a and 5b series showing all hydrogen atoms of the cyclohexane rings. Label each hydrogen atom as to whether it is axial or equatorial.
-
(a) Androsterone, a secondary male sex hormone, has the systematic name 3a-hydroxy- 5a-androstan-17-one. Give a three-dimensional formula for androsterone. (b) Norethynodrel, a synthetic steroid that...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...
-
Your portfolio has a beta of 1.17, a standard deviation of 14.3 percent, and an expected return of 12.5 percent. The market return is 11.3 percent and the risk-free rate is 3.1 percent. What is the...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...

Study smarter with the SolutionInn App


