For each of the following reactions, predict the product and draw the mechanism of its formation. a.
Question:
a.
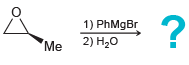
b.
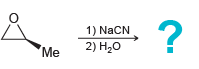
c.
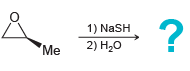
d.
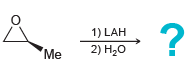
e.
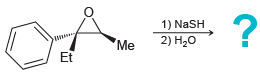
f.
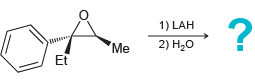
Transcribed Image Text:
1) PhMgBr 2) H20 Me 1) NaCN 2) H20 *Me
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
a b c ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product for each of the following reactions, (a) (b) (c) (d) N- 1) Excess Mel 2) Ag,0, H,0, heat
-
Predict the products of the following reactions: (a) (b) (c) (d) (e) (f) CI )LiAIH (2)H30 CI LiAIHO--Bu) (1) DIBALH OCH (2) H20 (I) excess CH2Mgl CI ) Ho (1) DIBAL-H (-78C) (2) H,0
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) (f) OH SOC, pyr OH HBr NaNH2 OH OH (1) TsCI, pyr (2) EtSNa Nal, H2SO OH
-
The top ten fiction books on The New York Times Best Sellers List on October 9, 2016, are listed. 1. The Girl on the Train 2. Home 3. The Kept Woman 4. Magic Binds 5. Commonwealth 6. The Light...
-
Rockwell Company owns a single restaurant which has a cantina primarily to seat patrons while they wait on their tables. This is considering eliminating the cantina and adding more dining tables....
-
Perform the indicated operations for the resulting complex numbers if the given changes are made in the indicated examples of this section. In Example 6, replace 2j with 2j and then find the roots....
-
Continuous process improvement. Processes can and must be improved to reduce cost and increase quality. (This topic was discussed in Chapter 14 and will not be covered in this chapter.)? LO.1
-
Consider heat transfer in a one-dimensional (radial) cylindrical coordinate system under steady-state conditions with volumetric heat generation. (a) Derive the finite-difference equation for any...
-
Assume the Mold Division of SPKY Corporation had the following results last year (in thousands). Management's target rate of return is 10% and the weighted average cost of capital is 7%. Its...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Calculate the P and T values for which Br2(g) is in a corresponding state to Xe(g) at 330. K and 72.0 bar.
-
For values of z near one, it is a good approximation to write z(P) = 1 + (z/P) T . P. If z = 1.00104 at 298 K and 1 bar, and the Boyle temperature of the gas is 155 K, calculate the values of a, b,...
-
Suppose that you are considering investing in a bank that is earning a higher ROE than most other banks. You learn that the bank has $300 million in capital and $5 billion in assets. Would you become...
-
2 4 . In the current year, Madison sold Section 1 2 4 5 property for $ 6 , 0 0 0 . The property cost $ 2 6 , 0 0 0 when it was purchased 5 years ago. The depreciation claimed on the property was $ 2...
-
Swifty Company purchased machinery on January 1, 2025, for $82,400. The machinery is estimated to have a salvage value of $8,240 after a useful life of 8 years. (a) Your answer is incorrect. Compute...
-
Currently, the unit selling price is $ 5 0 , the variable cost is $ 3 4 , and the total fixed costs are $ 1 0 8 , 0 0 0 . a . Compute the current break - even sales in units.
-
(1) The Mean Value Theorem states: Let f be continuous over the closed [a, b] and differentiable over the open interval (a, b). Then, there exists at least one point c E (a, b) such that: f(b) - f(a)...
-
Assume you are an Israeli investor; the symbol for the Israeli currency, the shekel, is ILS. You see that stock for Top Image has a bid price of ILS 17 and an ask price of ILS 19 in Israel, a bid...
-
Write an equation of the line passing through the given point and satisfying the given condition. (-2, 8); undefined slope
-
Outline a general process applicable to most control situations. Using this, explain how you would develop a system to control home delivery staff at a local pizza shop.
-
Identify the monomer units from which each of the following polymers is made, and tell whether each is a chain-growth or a step-growthpolymer. (b) +CF2-CFCI (c) NHCH2CH2CH2C (a) +CH2-0+o (d) (e)
-
Draw a three-dimensional representation of segments of the following polymers: (a) Syndiotactic poly acrylonitrile (b) Atactic poly (methyl methacrylate) (c) Isotactic poly (vinyl chloride)
-
Draw the structure of Kodel, a polyester prepared by heating dimethyl 1, 4-henzcnedicarhoxylate with 1, 4-bis (hydroxymethyl)cyclohexane. H? -CH2 1,4-Bis(hydroxymethyl)cyclohexane
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...

Study smarter with the SolutionInn App


