For each of the following substrates, determine whether an E1 process will require the use of an
Question:
a.
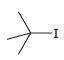
b.
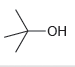
c.
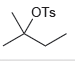
d.
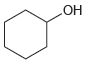
e.
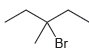
f.
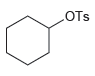
Transcribed Image Text:
-ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
a No the leaving group is not OH b Yes ...View the full answer

Answered By

Akshada Ghangale
I have my tutoring experience on other tutoring platform named chegg
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following situations? Determine whether a long or short hedge is appropriate. Justify your answer. a. A firm anticipates issuing stock in three months. b. An investor plans to buy a...
-
For each of the following studies, say whether you would use a t test for dependent means or a t test for independent means. (a) A researcher randomly assigns a group of 25 unemployed workers to...
-
Determine the relaxation time for each of the following medium: (a) Hard rubber ( = 1015 S/m, = 3.1o) (b) Mica ( = 1015 S/m, = 6o) (c) Distilled water ( = 104 S/m, = 80 o)
-
Cadmium is a highly toxic substance with lethal effects on the human body in concentrations greater than 4.4 x 10-mol/L. Cadmium (II) carbonate has a Kp of 5.2 x 10-12 at 25C. Calculate the...
-
Based on, Kerzner, Harold. (2013) Project Management: A Systems Approach to Planning, Scheduling, and Controlling, 11th Ed. John Wiley & Sons. ISBN 978-1118022276.(Chapter 17) and Kerzner, Harold....
-
Find the x-coordinate of the point on the curve y = x + 1/x - 1where the gradient is 0.
-
Statistical significance. A study, mandated by Congress when it passed No Child Left Behind in 2002, evaluated 15 reading and math software products used by 9424 students in 132 schools across the...
-
Southlake Corporation issued $900,000 of 8% bonds on March 1, 20X1. The bonds pay interest on March 1 and September 1 and mature in 10 years. Assume the independent cases that follow. Case AThe bonds...
-
Xx) P9-42 (similar to) Question Help Global Energy Saver (GES), a producer of energy-efficient light bulbs, expects that demand will increase markedly over the next decade. Due to the high fixed...
-
There are 2 shinobis with chakra levels 5 and 10 respectively and the desired sum of chakra levels is utmost 15 Starting with ke0, suy of chakra levels after attack max(5-0,0) + max(10- 0,0) 5+10 15....
-
What are fossil fuels?
-
How are coal, oil, and natural gas formed?
-
If a force of 16.7 N is pressed against an area of 2.44 m2, what is the pressure in pascals?
-
Before beginning a study investigating the ability of the drug heparin to prevent bronchoconstriction, baseline values of pulmonary function were measured for a sample of 12 individuals with a...
-
which of the following (list all that apply) are advantages of a balanced binary search tree over an unbalanced one: 1. it requires less memory 2. it's faster to move from node to node 3. it's faster...
-
6) Do you find conditional probability problems challenging? Have you tried watching the videos on canvas and has it helped?
-
1. Determine the cost of heating 3 gallons of water (water weighs 8.33L per gallon ) at a room temperature of 22 degrees Celsius to the boiling point of 100 degrees Celsius at the energy rating of...
-
Writer One Inc. manufactures ball point pens that sell at wholesale for $0.80 per unit. Budgeted production in both 2018 and 2019 was 16,000 units. There was no beginning inventory in 2018. The...
-
Factor out the greatest common factor. 4 + 20 z
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
How strong a base would you expect a Grignard reagent to be? Look at Table 8.1, and then predict whether the following reactions will occur as written.?(The p K a of NH 3 is 35.) (a) CH 3 MgBr + H ?...
-
How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a deuteratedcompound? Br , CHH-H CHCH2CH
-
How would you carry out the following transformations using an organo copper coupling reaction? More than one step is required in eachcase. (a) "CH (b) HH2CH2CHBr CH3CH2CH2CH2CH2CH2CH2CH3 (c)...
-
September 23 for $1,050 each. On December 24 , it sold one of the diamonds that was purchased on July 9 . Using the specific identification method, its ending inventory (after the December 24 sale)...
-
Madsen Motors's bonds have 13 years remaining to maturity. Interest is paid annually, they have a $1,000 par value, the coupon interest rate is 8%, and the yield to maturity is 10%. What is the...
-
Builder Products, Incorporated, uses the weighted - average method in its process costing system. It manufactures a caulking compound that goes through three processing stages prior to completion....

Study smarter with the SolutionInn App


