How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a
Question:
How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a deuteratedcompound?
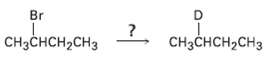
Transcribed Image Text:
Br роно, CHзснсH-сHз CHзCнсH2CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Just as Grignard reagents react with prot...View the full answer

Answered By

Sourabh Mahajan
I have an experience of 5 months in teaching. I think all students were satisfied with my teaching style and many become my fan. I enjoyed doubt solving and i also motivate them for future.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A deuteron (nucleus of deuterium atom consisting of a proton and a neutron) with speed 14.9 km/s collides elastically with a neutron at rest. Use the approximation that the deuteron is twice the mass...
-
How many hydrogens are replaced by deuterium when each of the following compounds is treated with NaOD in D2O? a. 3-methylcyclopentanone b. 3-methylhexanal
-
If you wanted to prepare a solution of CO in water at in which the CO concentration was 2.5 mM, what pressure of CO would you need to use? (See Figure 13.19.)
-
Why does the magnetization current impose an upper limit on the voltage applied to a transformer core?
-
Contributions to the disaster relief fund for a national charity average around $1 million a month and do not exhibit trend or seasonal effects. Data on the most recent 12 months is given in the...
-
Performance curves for an operating centrifugal pump are shown below in both conventional units and in dimensionless form. The pump is used to pump water at maximum efficiency at a head of 90 m....
-
Define the different types of brand extensions. LO3
-
When would the sum of the break-even quantities for each of a companys products not be the break-even point for the company as a whole?
-
An 8% coupon bond with 10-year maturity making annual payments is selling today at $1,050. The par value is $1,000. The bond is callable in 5 years and the call price is $1,120. The yield to call on...
-
Kurt and Wyatt are forming a partnership to develop a theme park near Carlson City, Florida. Kurt contributes cash of $3,000,000 and land with a current market value of $10,500,000. When Kurt...
-
How strong a base would you expect a Grignard reagent to be? Look at Table 8.1, and then predict whether the following reactions will occur as written.?(The p K a of NH 3 is 35.) (a) CH 3 MgBr + H ?...
-
How would you carry out the following transformations using an organo copper coupling reaction? More than one step is required in eachcase. (a) "CH (b) HH2CH2CHBr CH3CH2CH2CH2CH2CH2CH2CH3 (c)...
-
A firm will break even (no profit and no loss) as long as revenue just equals cost. The value of x (the number of items produced and sold) where C(x) = R(x) is the break-even point. Assume that each...
-
(ii) State Wilkie's updating equation in respect of the force of inflation and explain carefully what each of the components of the equation represents. State also which type of time series process...
-
Compute the double integral D x y dA over the domain D indicated as 0 x 5, x y 2x + 3. (Use symbolic notation and fractions where needed.) f(x, y) A = D
-
4. (10 points) A researcher believes that length of time spent listening to classical music increases memory for previously learned material. She has 4 groups of 5 subjects listen to either 10 min.,...
-
We find a binary system consisting of a 1 solar mass star, still in its main sequence phase, and a white dwarf. Assume both stars formed at the same time and that they did not significantly influence...
-
The equity sections from Atticus Group's 2015 and 2016 year-end balance sheets follow. Stockholders Equity (December 31, 2015) Common stock $6 par value, 50,000 shares authorized, 35,000 shares...
-
Identify the manager or supervisors role in new-employee orientation.
-
Banner Company acquires an 80% interest in Roller Company for $640,000 cash on January 1, 2013. The NCI has a fair value of $160,000. Any excess of cost over book value is attributed to goodwill. To...
-
Which statement is true? (a) A redox reaction involves either the transfer of an electron or a change in the oxidation state of an element. (b) If any of the reactants or products in a reaction...
-
Use your results from Problem 3-25 to complete the following table. Each entry shows the positions of two groups arranged as shown. For example, two groups that are trans on adjacent carbons...
-
Draw the two chair conformations of each of the following substituted cyclohexanes. In each case, label the more stable conformation. (a) cis-1-ethyl-2-methylcyclohexane (b)...
-
Name the following compounds. Remember that two up bonds are cis; two down bonds are cis; one up bond and one down bond are trans. (a) (b) (c) (d) (e) (f) . CH H,C , CH CH H,C CH CH CH, H,C
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


