How many signals would you expect in the 13 C NMR spectrum of each of the compounds
Question:
In Problem 16.34
How many signals would you expect in the 1H NMR spectrum of each of the following compounds:
(a)
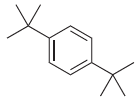
(b)
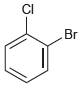
(c)
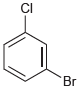
(d)
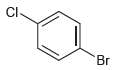
(e)
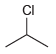
(f)
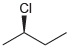
Transcribed Image Text:
CI Br ū-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (16 reviews)
a 4 ...View the full answer

Answered By

Salmon ouma
I am a graduate of Maseno University, I graduated with a second class honors upper division in Business administration. I have assisted many students with their academic work during my years of tutoring. That has helped me build my experience as an academic writer. I am happy to tell you that many students have benefited from my work as a writer since my work is perfect, precise, and always submitted in due time. I am able to work under very minimal or no supervision at all and be able to beat deadlines.
I have high knowledge of essay writing skills. I am also well conversant with formatting styles such as Harvard, APA, MLA, and Chicago. All that combined with my knowledge in methods of data analysis such as regression analysis, hypothesis analysis, inductive approach, and deductive approach have enabled me to assist several college and university students across the world with their academic work such as essays, thesis writing, term paper, research project, and dissertation. I have managed to help students get their work done in good time due to my dedication to writing.
5.00+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many signals would you expect in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) (d) (e) (f) CI Br -
-
A compound with molecular formula C 17 H 36 exhibits a 1 H NMR spectrum with only one signal. How many signals would you expect in the 13C NMR spectrum of this compound?
-
A compound with molecular formula C 8 H 18 exhibits a 1 H NMR spectrum with only one signal. How many signals would you expect in the 13 C NMR spectrum of this compound?
-
Exporting files into binary files is done in O Logical files O Physical backup O Physical full backup O Physical partial backup L
-
1. By 2020 how many smartphones will be available globally? 2. Who are the two companies that generate the biggest revenue from mobile ads? 3. What does PlaceIQ do for businesses? 4. For what purpose...
-
Suppose you have an oracle, OM(s), that correctly predicts the opponents move in any state. Using this, formulate the definition of a game as a (single-agent) search problem. Describe an algorithm...
-
Describe ethically neutral leadership. AppendixLO1
-
State Senator Bowdler convinced the legislature of State Z to pass a law requiring all professors to submit their class notes and transparencies to a board of censors to be sure that no lewd...
-
Agile' is a term that is gaining in popularity in strategy. Which of the following statements is true? Agile approaches assume that strategic decisions are reversible (if something is initiated and...
-
What is the Hamming distance for each of the following codewords? a. d (10000, 00000) b. d (10101, 10000) c. d (00000, 11111) d. d (00000, 00000)
-
Because of crop failures last year, the San Joaquin Packing Company has no funds available to finance its canning operations during the next six months. It estimates that it will require $1,200,000...
-
How would you distinguish between the following compounds using 13 C NMR spectroscopy?
-
(a) Make a graph showing retention times of peaks 6, 7, and 8 in Figure 24-12 as a function of %acetonitrile (%B). Predict the retention time of peak 8 at 45% B. (b) In Figure 24-12, tm = 2.7 min....
-
Air at \(1 \mathrm{~atm}\) and \(25^{\circ} \mathrm{C}\) flows in a 4-cm-diameter glass pipe at a velocity of \(7 \mathrm{~m} / \mathrm{s}\). The friction factor for this flow is (a) 0.0266 (b)...
-
Find the regime of the boundary layer at the location of \(0.1 \mathrm{~m}\) from the leading edge of an aerofoil, if the airspeed is \(80 \mathrm{~ms}^{-1}\), and air condition is at that of sea...
-
``Nuclear Deterrence,'' by Harvey A . Smith, UMAP327. The author analyzes the stability of the arms race, assuming objectives similar to those suggested by General Taylor. The module develops...
-
A saturated vapor feed at \(1000.0 \mathrm{kmol} / \mathrm{h}\) of methanol \((5.0 \mathrm{~mol} \%)\) and water \((95.0 \mathrm{~mol} \%)\) is fed to a distillation column with 18 stages plus a...
-
Use the partition functions in Equations 1.98, 1.99, and 1.100 to find the translational, rotational, and vibrational contributions to the average energy of a diatomic molecule. Compare each result...
-
State the inverse action or actions. Opening a window
-
One Way Cellular accountants have assembled the following data for the year ended September 30, 2014: Prepare the operating activities section using the indirect method for One Way Cellulars...
-
Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a monoanion and one for the second dissociation into a dianion. For oxalic acid, HO2CCO2H, the first...
-
The pKa of p-cyclopropylbenzoic acid is 4.45. Is cyclopropylbenzene likely to be more reactive or less reactive than benzene toward electrophilic bromination? Explain.
-
Rank the following compounds in order of increasing acidity. Dont look at a table Of PKa data to help with your answer. (a) Benzoic acid, p-methyl benzoic acid, p-chlorohenzoic acid (b)...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


