How would you use IR spectroscopy to distinguish between the following pairs of compounds? (a) (b) N.
Question:
(a)
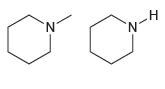
(b)
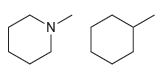
Transcribed Image Text:
N.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
a The second compound will have an NH stretching signal between 3300 ...View the full answer

Answered By

Sandip Agarwal
I have an experience of over 4 years in tutoring. I have solved more than 2100 assignments and I am comfortable with all levels of writing and referencing.
4.70+
19+ Reviews
29+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How might you use IR spectroscopy to distinguish among the three isomers 1 -butyne, 1, 3-hutadiene, arid 2-butyne?
-
How could you use IR spectroscopy to distinguish between the isomers 1-hexyne and 1,3-hexadiene?
-
How could you use IR spectroscopy to distinguish between the following pairs of isomers? a. b. c. (CH3CH2)3N and (CH3CH2CH2)2NH 0 CH.CCH,CH, and CH CHCH-CH, CH3 CHCHO and CH CHOCH
-
Define variable. Also discuss variable initialization.
-
The complexity of interpersonal relationships increases dramatically as the size of a group increases. Determine the numbers of different two-person relationships in groups of people of sizes (a) 3,...
-
One problem with all exponential growth models is that nothing can grow exponentially forever. Describe factors that might limit the size of a population.
-
What is the difference between the par value and the call price of a share of preferred stock? AppendixLO1
-
Last year Wyeth Company recorded an impairment on an asset held for use. Recent appraisals indicate that the asset has increased in value. Should Wyeth record this recovery in value?
-
calculate the intrinsic value of common stock for xyz at January 1,year 1 given the following: Book value $50 Predicted earnings per share years 1 through 5 respectively $10, $8, $12, $15, and $10...
-
According to the American Metal Markets Magazine, the spot market price of U.S. hot rolled steel recently reached $580 per ton. Less than a year ago this same ton of steel was only $260. A number of...
-
A compound with molecular formula C 5 H 13 N exhibits three signals in its proton NMR spectrum and no signals above 3000 cm 1 in its IR spectrum. Draw two possible structures for this compound.
-
Discuss opportunities and risks associated with each brothers proposal.
-
A thin-walled double-pipe, counter-flow heat exchanger is to be used to cool oil (c p = 0.525 Btu/lbmF) from 300F to 105F at a rate of 5 lbm/s by water (c p = 1.0 Btu/lbmF) that enters at 70F at a...
-
1. (5 pts) Given y[n]= 2y[n-1] and y[0]=2, Write MATLAB code to calculate and plot y for 0
-
F ( t ) = t 4 + 1 8 t 2 + 8 1 2 , g ( t ) = ( t + 3 ) / 3 ; find ( f o g ) ( 9 )
-
How did they calculate allocated cost FLIGHT A FLIGHT 350 615 FLIGHT 3 1 Go GALS 20 G EXISTING SCHEME, DETERMINE THE OVE OR FLIGHTS A, B, AND C. 2 ED AT 7.00 PER K1.00 OF PILOT SALAF TOTAL NON-SALARY...
-
High Tech ManufacturingInc., incurred total indirect manufacturing labor costs of $540,000. The company is labor-intensive. Total labor hours during the period were 5,000. Using qualitativeanalysis,...
-
Start with AS/AD and IS/MP in full employment equilibrium. Assume the is a massive positive aggregate demand shock. How would this affect AS/AD and IS/MP and prices and output relative to the full...
-
The function f(x) = -0.00002x 3 + 0.008x 2 - 0.3x + 6.95 models the number of annual physician visits, f(x), by a person of age x. Graph the function in a [0, 100, 5] by [0, 40, 2] viewing rectangle....
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed. (a) Cyclo-hexane (b)...
-
When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of...
-
The chlorination of pentane gives a mixture of three mono-chlorinated products. (a) Draw their structures. (b) Predict the ratios in which these mono-chlorination products will be formed, remembering...
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


