Identify the base you would use for each of the following transformations. (a) (b) 1) ? 2)
Question:
(a)
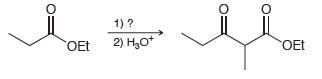
(b)
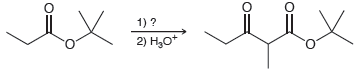
Transcribed Image Text:
1) ? 2) Н,о OEt OEt 1) ? 2) H,o*
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
a N...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify whether you would use dilute sulfuric acid or concentrated sulfuric acid to achieve each of the following transformations. In each case, explain your choice. a. b. [H,SO,] - . [H,SO,] + H20
-
Identify the reagents that you would use to accomplish each of the following transformations (you will also need to use reactions from previous chapters). (a) (b) (c) Br Br OH
-
Identify the reagents that you would use to accomplish each of the following transformations (you will need to use reactions from previous chapters). (a) (b) (c) (d) OEt CI
-
Mr. Ajay's trial balance is as follows. Trial Balance for the year ended 31st March, 2017 Amt Debit Balances To Opening Stock To Purchases To Wages To Investment To Carriage outwards To Printing and...
-
The Distribution Point plans to save $2,000 a month for the next 3 years for future emergencies. The interest rate is 4.5 percent compounded monthly. The first monthly deposit will be made today....
-
A very conscientious and quality driven tire retailer (Todd Witt Tires) is in the process of determining which tire supplier to use to purchase tires, which will be sold to end customers. Todd Witt...
-
Company X has the following bonds outstanding: Bond A Bond B Coupon Maturity 8% 10 years Coupon Variablechanges annually to be comparable to the current rate Maturity 10 years Initially, both bonds...
-
As a result of a recent survey of students at the University of South Wisconsin, it was determined that the university owned bookstore currently has 40% of the market. The other three bookstores,...
-
Equivalent Units of Conversion Costs The Rolling Department of Oak Ridge Steel Company had 4,433 tons in beginning work in process inventory (80% complete) on July 1. During July, 34,100 tons were...
-
The balcony located on the third floor of a motel is shown in the photo below. It is constructed using a 100-mm thick concrete (plain stone) which rests on the four simply supported floor beams, two...
-
When 2,6-heptanedione is heated in the presence of aqueous sodium hydroxide, a condensation product with a six-membered ring is obtained. Draw the product and show a mechanism for its formation.
-
Consider the following model: Y t = 1 + 2 X t + 3 X t 1 + 4 X t 2 + 5 X t 3 + 6 X t 4 + u t where Y = consumption, X = income, and t = time. The preceding model postulates that consumption...
-
What is the PCAOB and what is its authority?
-
A new partner C is invited to join in the AB partnership. Currently, A's and B's capital are $540,000 and $100,000, respectively. According to their profit and loss sharing contract, partner A and B...
-
The two tanks shown are connect through a mercury manometer. What is the relation between ???? and ? water Az water Ah
-
1. After reading about the types of rights that prisoners have while incarcerated, which of these rights, if any, should be reduced or diminished? Why? 2. In the same way, what rights do you believe...
-
According to the Socratic view of morality summarized by Frankena, is a person brought up by immoral parents in a corrupt society capable of making correct moral judgements? Why or why not? Do you...
-
Loma Company manufactures basketball backboards. The following information pertains to the company's normal operations per month: Output units15,000 boards Machine-hours4,000 hours Direct...
-
Boyles law states that when gas is compressed at a constant temperature, the pressure P and volume V of a given sample satisfy the equation PV = C, where C is constant. Suppose that at a certain time...
-
Reconsider Prob. 1474. In order to drain the tank faster, a pump is installed near the tank exit as in Fig. P1475. Determine how much pump power input is necessary to establish an average water...
-
Give the structure of the product formed when 3-methylhexanoic acid is heated with a large excess of ethanol (as solvent) with a sulfuric acid catalyst.
-
(a) Using the principle of microscopic reversibility, give a detailed mechanism for the acid-catalyzed hydrolysis of methyl benzoate (structure in Eq. 20.16, p. 965) to benzoic acid and methanol. (b)...
-
(a) Using the principle of microscopic reversibility, give a detailed mechanism for the acid-catalyzed hydrolysis of methyl benzoate (structure in Eq. 20.16, p. 965) to benzoic acid and methanol. (b)...
-
What is the Macaulay duration of a bond with a coupon of 6.6 percent, seven years to maturity, and a current price of $1,069.40? What is the modified duration? (Do not round intermediate...
-
"Tell me something you know today that you did not know yesterday" about 3D Animation Justify by citing 2 or more resources from this course.
-
Warrants exercisable at $20 each to obtain 50,000 shares of common stock were outstanding during a period when the average market price of the common stock was $25. Application of the treasury stock...

Study smarter with the SolutionInn App


