Identify the number of chirality centers in each of the following compounds: (a) (b) (c) `NH2 N'
Question:
(a)
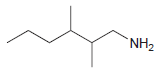
(b)
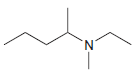
(c)
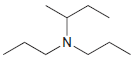
Transcribed Image Text:
`NH2 N'
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
a T...View the full answer

Answered By

Moses mwangi
With prior writing experience, be sure that I will give a great grade, If not an A+, it will be something close to this. My reviews speaks it all, Try me!!
4.80+
78+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Name the following compounds: a. b. c. d. CH,CH OH Cl Br Br. CH,CH CH3
-
Predict the product(s) for each of the following reactions. In each case, make sure to consider the number of chirality centers being formed. a. b. c. d. e. f. Os0, (catalytic) NMO 1) OsO, 2) NaHSO,...
-
Name the following compounds: a. b. c. d. e. f. Br
-
Determine the degrees of freedom under the following conditions: (a) Tl-20 wt% Pb at 325 C and 400 C; (b) Tl-40 wt% Pb at 325 C and 400 C; (c) Tl-90 wt% Pb at 325 C and 400 C. Refer to the phase...
-
What is the internal rate of return for a project that has a net investment of $75,000 and the following net cash flows: Year 1 = $15,000; Year 2 = $20,000; Year 3 = $25,000; Year 4 = $30,000?
-
Find the number of terms in each arithmetic sequence. 3 21 3, 4 4 ., 12
-
Gin is retiring from the partnership, and the partners agreed that she should receive $200,000 cash as payment in full for her share of partnership assets. If the goodwill implied by the settlement...
-
An article by J. J. Pignatiello, Jr. and J. S. Ramberg in the Journal of Quality Technology (Vol. 17, 1985, pp. 198- 206) describes the use of a replicated fractional factorial to investigate the...
-
Claudine Corporation will deposit $ 4 , 5 0 0 into a money market account at the end of each year for the next four years. How much will accumulate by the end of the fourth and final payment if the...
-
When Marge Simpson, PA, audited the Candle Company inventory, a random sample of inventory types was chosen for physical observation and price testing. The sample size was 80 different types of...
-
Do these findings surprise you? Why or why not? How might you apply what you have learned about social media and social media marketing from this chapter to your own workplace ethics?
-
1. Do you agree with P&G that online marketing will save money without hurting its brands in the market place? Why or why not? 2. What non-financial advantages of online marketing can P&G try to...
-
This happens if a PDE involves derivatives with respect to one variable only (or can be transformed to such a form), so that the other variable(s) can be treated as parameter(s). Solve for u = u(x,...
-
7. This is a question about electromagnetic waves. (a) Starting from Maxwell's equations in a vacuum show that the electric field E and magnetic field B obey wave equations and identify the velocity...
-
Participate in workplace health and safety Third- party report Task 1: Case scenario: Workplace hazard collection and risk control form You are required to review this workplace inspection form and...
-
The red curve is the position-time x-t graph for the ladybug. Each tick mark on the time axis of the graph marks off 0.5 s. Note: you can hit reset graph and graph again to watch the graph form again...
-
Oma's Bakery is thinking about replacing the convection oven with a new, more energy-efficient model. Information related to the old and new ovens follows: (Click the icon to view the information...
-
One could argue that substantial travel for work is an undesirable characteristic of any job. What would the theory of compensating differentials predict about the relative wages of a sales position...
-
Exercises 101103 will help you prepare for the material covered in the first section of the next chapter. -x 2 - 2x + 1 = 0
-
What are some of the possible sources of information about a company that could be used for determining the companys competitive stance?
-
Ammonia appears in Table 1-5 both as an acid and as a conjugate base.
-
Write equations for the following acid-base reactions. Use the information in Table 1-5 to predict whether the equilibrium will favor the reactants or the products. (a) HCOOH + -CH (b) CH3COO- +...
-
Solved Problem 1-5(c) showed protonation of the double-bonded oxygen in acetic acid. Show the product of protonation on the other (-OH) oxygen. Explain why protonation of the double-bonded oxygen is...
-
Ted and his partners have contracted to purchase the franchise nights worth 561 000 to open and operate a specialty pizza restaurant called Popper with a renewable agrement, the partners have agreed...
-
Your answer is partially correct. Martin Company's chief financial officer feels that it is important to have data for the entire quarter especially since their financial forecasts indicate some...
-
Kellog Corporation is considering a capital budgeting project that would have a useful life of 4 years and would love testing 5156.000 in equipment that would have zeto salvage value at the end of...

Study smarter with the SolutionInn App


