Identify the reagents necessary to prepare each of the following compounds using a Wittig reaction: (a) (b)
Question:
(a)
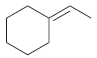
(b)
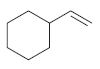
(c)
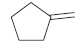
(d)
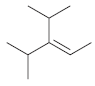
(e)
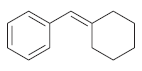
Transcribed Image Text:
-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
a ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the reagents you would use to prepare each of the following compounds via a Diels-Alder reaction: (a) (b) (c) (d) (e) (f) (g) (h) COOH COOH
-
Identify the reagents necessary to achieve each of the following transformations: Br Br - " Br Br "Br
-
Identify the reagents necessary to achieve each of the following transformations: Br Br Br Br Br
-
Suppose you were required to use a micrometeorite shield no more than 0.01 meters thick. What would be the required toughness of the material from which that shield was made if the shield must...
-
Justify paying $200,000 today for an investment that will generate $15,000 annual benefits to its owner into perpetuity.
-
Your manager has also requested that you grant the GG Studios team access to the Secure Labs on Demand NAS server, to ensure their files are properly backed up. Using the TrueNAS server at...
-
Measuring physical fitness. You want to measure the physical fitness of college students. Give an example of a clearly invalid way to measure fitness. Then briefly describe a measurement process that...
-
Suppose that the size of pebbles in a river bed is normally distributed with mean 12.1mm and standard deviation 3.2 mm. A random sample of 9 pebbles are measured. Let denote the average size of the...
-
Ironwood Company manufactures a variety of sunglasses. Production information for its most popular line, the Clear Vista ( CV ) , follows: \ table [ [ , Per Unit ] , [ Sales price,$ 5 0 . 5 0
-
Bonds 1. Municipal Bonds - Municipal bonds are haircut per Exhibit 1 based on both their time to maturity and scheduled maturity at date of issue. 2. Corporate Bonds - Corporate bonds are haircut...
-
Identify the reagents necessary to accomplish each of the transformations below: (a) (b) OH HO NH2
-
Consider the structure of beta-carotene, mentioned earlier in this chapter: Design a synthesis of beta carotene using the compound below as your only source of carbon atoms: B-carotene Br
-
Trivoli Industries plans to issue some $100 par preferred stock with an 11 percent dividend. The stock is selling on the market for $97.00, and Trivoli must pay flotation costs of 5 percent of the...
-
Consider the following thermochemical equation: 2 Na 2 O 2 (s) + 2 H 2 O(l) 4 NaOH(s) + O 2 (g) H = -126 kJ Calculate the enthalpy change when 41.5 g of Na 2 O 2 with water?
-
Newton's Laws Introduction Problems
-
Mr. A and B agreed to start a business agreed to share profit and loss based the condition that will profit only when there is profit in excess of BD 10,000 this from of business is called as:...
-
L= {a'e"b"d' | i=1+m and l,m,n 20] a. Write at least 10 strings of the above language in increasing order of string length. b. Write Context Free Grammar (CFG) for the above language.
-
The Beta Co. shows the following results of operation on Dec. 31. Variable cost Fixed costs Direct materials P512,500 Direct labor 575,000 Manufacturing overhead 400,000 P212,500 For the year then...
-
Solve each system. -x + 2y + 6z = 2 + 3x + 2y + 6z = 6 x + 4y - 3z = 1
-
Determine the optimal use of Applichem's plant capacity using the Solver in Excel.
-
The pKa of the anilinium ion (C6H5NH3) is 4.63. On the basis of this fact, decide whether aniline (C6H5NH2) is a stronger or weaker base than methylamine.
-
Predict the outcome of the following reaction. -NH2
-
Write condensed and bond-line structural formulas for all of the constitutional isomers with the molecular formula C7H16. (There are a total of nine constitutional isomers.)
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


