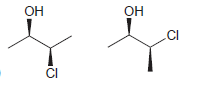
Identify whether each of following pairs of compounds are enantiomers or diastereomers: a. b. c. d. e.
Question:
a.
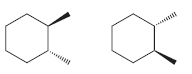
b.
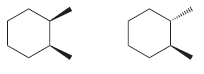
c.
d.

e.

f.
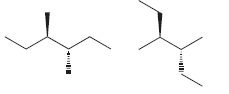
Transcribed Image Text:
OH OH .CI CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a Enantiomers b Di...View the full answer

Answered By

BANOTHU RAJU
I am teacher.
and tutor in Chegg , course Hero
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Indicate whether each of the following pairs of compounds are identical or are enantiomers, diastereomers, or constitutional isomers: a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. H C) and H H H CI...
-
Each of the following pairs of compounds undergoes a Bronsted acid-base reaction for which the equilibrium lies to the right. Give the products of each reaction, and identify the acid, the base, the...
-
For each of the following pairs of compounds, identify one IR absorption band that could be used to distinguish between them: a. b. c. d. e. f. g. h. i. cis-2-butene and trans-2-butene j. CH3CH2CH2OH...
-
Find dy/dx for the following functions. y = sin x + cos x
-
Write some most important questions on Ms word from the Book Chapter 12.
-
Find the third Taylor polynomial of x 2 at x = 3.
-
Underabsorption of overheads means that actual overheads are more than absorbed overheads.
-
A ticket from Indianapolis to Orlando on Deleast Airlines sells for $150. The plane can hold 100 people. It costs Deleast $8000 to fly an empty plane. Each person on the plane incurs variable costs...
-
1 K M N 0 Q R Notes Revenues is driven by the growth rate Gross Protits is calculated as Ravenues multiplied by Gross Margin 1700 Opex is calculated as Revenues multiplied by OpEx Percent Revenues ....
-
Chateau des Charmes Wines bought special corks for its wines from Sabate USA, a wholly owned subsidiary of Sabate France. The corks were not supposed to cause wines to be spoiled by cork taint. The...
-
The specific rotation of l -alanine in water (at 25C) is +2.8. A chemist prepared a mixture of l -alanine and its enantiomer, and 3.50 g of the mixture was dissolved in 10.0 mL of water. This...
-
Classify each of the following compounds below as cis, trans, or not stereo-isomeric: , ,
-
Allen Products, Inc., wants to do a scenario analysis for the coming year. The pessimistic prediction for sales is $900,000; the most likely amount of sales is $1,125,000; and the optimistic...
-
Juanita Poblamo makes large ceramic pots for use in outdoor landscape. She currently has two models, one square and the other round. Because of the size of Juanitas creations, only one pot can be...
-
EPI educational products are currently sold without any supplemental materials. The company is considering the inclusion of instructional materials such as an overhead slide presentation, potential...
-
EPI is considering eliminating a product from its ToddleTown Tours collection. This collection is aimed at children one to three years of age and includes tours of a hypothetical town. Two products,...
-
Suppose we estimate the model y i = + u i , where u i N [ 0 , i 2 ] . (a) Show that the OLS estimator of simplifies to ^ = y . (b) Hence directly obtain the variance of y . Show that this...
-
This question presumes access to software that allows NLS and ML estimation. Consider the gamma regression model of Exercise 5-2. An appropriate gamma variate can be generated using \(y=-\lambda \ln...
-
In Exercises 8194, begin by graphing the absolute value function, f(x) = |x|. Then use transformations of this graph to graph the given function. g(x) = -x + 4 + 1
-
Subtract the polynomials. (-x+x-5) - (x-x + 5)
-
Indicate reagents that would accomplish each of following transformations. More than one reaction may be necessary in some cases. (a) (b) (c) (d) (e) (f) OCH HO CI Cl OCH3 NH OH ENH,CI
-
Which acid of each pair shown here would you expect to be stronger? (a) (b) (c) (d) (e) HO HO 10 HO HO 3 10 F O or OH OH or MeJN OH OH or CF
-
Write structural formulas for the major organic products from each of the following reactions. (a) (b) (c) (d) (e) CI CH CH.SH NH2 (excess Cl OH H SO, (cat.) HO AlCl3
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


