Classify each of the following compounds below as cis, trans, or not stereo-isomeric: , ,
Question:
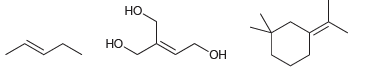
Transcribed Image Text:
но, Но но, НО Он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
trans HO H...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Classify each of the following compounds as an alkane, alkene, alkyne, alcohol, aldehyde, amine, and so forth. (a) (b) CH3-C¡CH (c) (d) (e) (f) Obtained from oil of cloves -2. Sex attractant...
-
Determine whether each of the following compounds is a cis isomer or a trans isomer a. b. c. d. e. f. Cl Br CH3 CH3 Br Br CH3 Cl CH3 CH3 CH
-
Draw both chair conformations for each of the following compounds. In each case, identify the more stable chair conformation: (a) Methylcyclohexane (b) Trans-1,2-Diisopropylcyclohexane (c)...
-
Evaluate the derivatives of the following functions. (z) = cot -1 z
-
Read the case study titled "Pre-Launch decisions which influence innovation success". Write a paper in which the following items are addressed. Discuss the necessity of short-term and long-term...
-
Determine the third Taylor polynomial of the given function at x = 0. f(x)=4x+1
-
Basis of apportionment of stores service expenses is the value of the material consumed.
-
Clarke, Inc., manufactures door panels. Suppose Clarke is considering spending the following amounts on a new total quality management (TQM) program: Clarke expects the new program would save costs...
-
Problem 2. Merchandising General Journal Entries (30 points) epare general journal entries to record the following merchandising transactions of Margin Company, which applies the perpetual inventory...
-
consider the following continuously operating job shop. Inter-arrival. Inter-arrival time of job are distribute as follows: Processing times for jobs are normally distributed, with mean 50 minutes...
-
Identify whether each of following pairs of compounds are enantiomers or diastereomers: a. b. c. d. e. f. OH OH .CI CI
-
For each of the following pairs of compounds, determine the relationship between the two compounds: a. b. c. d. e. f. g. h. i. j. k. l. Br Br
-
The limit order book for a security is as follows: The specialist receives the following, in order: Market order to sell 300 shares Limit order to buy 100 shares at 25.38 Limit order to buy 500...
-
The following information appears in the records of Poco Corporation at year-end: a. Calculate the amount of retained earnings at year-end. b. If the amount of the retained earnings at the beginning...
-
For the following four unrelated situations, A through D, calculate the unknown amounts appearing in each column: A B D Beginning Assets... $38,000 $22,000 $38,000 ? Liabilities.. 22,000 15,000...
-
On December 31, John Bush completed his first year as a financial planner. The following data are available from his accounting records: a. Compute John's net income for the year just ended using the...
-
Statement of Stockholders' Equity and Balance Sheet The following is balance sheet information for Flush Janitorial Service, Inc., at the end of 2019 and 2018: Required a. Prepare a balance sheet as...
-
Petty Corporation started business on January 1, 2019. The following information was compiled by Petty's accountant on December 31, 2019: Required a. You have been asked to assist the accountant for...
-
In Exercises 7192, find and simplify the difference quotient f(x +h)-f(x) h -, h = 0
-
In Exercises 105108, evaluate each expression without using a calculator. log(ln e)
-
Write structural formulas for the major organic products from each of the following reactions. (a) (b) (c) (d) (e) (1) KCN (2) H20, H,SO, (cat.) CN (1) DIBAL-H (1) CH,MgBr (2) H,O NH H,0, H.SO, (cat)...
-
Provide a detailed mechanism for each of the following reactions. (a) (b) (c) H,so, (cat.) OCH3 + CH OH (excess) 0 EtNH2 (excess) Cl NH H,O, H,SO, (cat.) OH
-
On heating, cis-4-hydroxycyclohexanecarboxylic acid forms a lactone but trans-4-hydroxycyclohexanecarboxylic acid does not. Explain.
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


