In Chapter 10, we will see that an acetylide ion (formed by treatment of acetylene with a
Question:
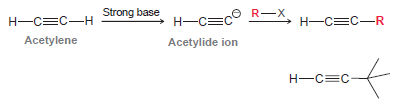
This reaction provides a useful method for making a variety of substituted alkynes. Determine whether this process can be used to make the following alkyne. Explain your answer.
Transcribed Image Text:
Strong base O R-X Н—С—С Н—СС—R Н—СС-н Acetylene Acetylide ion Н—с—С
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
No Preparation of this compo...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Can a buffer be made by combining a strong acid with a strong base? Why or why not?
-
A large slab of concrete, 5 8 0.3 m is used as a thermal storage mass in a solar-heated house. If the slab cools overnight from 23C to 18C in an 18C house, what is the net entropy change associated...
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
A car costs 12,000. It will be kept for three years, and then sold for 3,000. Calculate the depreciation for each year using (a) the reducing balance method, using a depreciation rate of 35 per cent,...
-
What issues confront the company as of mid-2012? What should Starbuck's management be worried about?
-
Find the equation of each of the lines with the given properties. Sketch the graph of each line. Perpendicular to line with slope of 3; passes through (1,2).
-
What is escalation clause?
-
The chief accountant for Dickinson Corporation provides you with the following list of accounts receivable that were written off in the current year: Dickinson Corporation follows the policy of...
-
. Correa Corp., which began business at the start of the current year, had the following data: Planned and actual production: 20,000 units Sales: 17,000 units at $15 per unit Production costs: o...
-
The following are the balances of the assets, liabilities, and equity of Fitness Fanatics at March 31, 2016: Requirements 1. What type of business organization is Fitness Fanatics? 2. Prepare the...
-
Identify the configuration of each chirality center in the following compounds: a. b. c. d. e. f. g. h. i. Et OH Me NH2
-
Predict the product(s) obtained when each of the following compounds is treated with chloromethane and aluminum trichloride. Some of the compounds might be unreactive. For those that are reactive,...
-
Evaluate the integral. r2 dp. (x + 1)? J1
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
3.6. Explain and discuss the potential benefits to be gained by using blade twist, plan- form taper, low solidity, large radius, and low rotational speed for the main rotor of a heavy lift helicopter...
-
2. A VRM (Voltage Regulator Modul) is used to supply the voltageto the CPU of a computer. In the new generation of microprocessors,whose power consumption is 100W, the input voltage to the VRM is12V...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
In Problems 3944, the slope and a point on a line are given. Use this information to locate three additional points on the line. Slope -2; point (-2, -3)
-
The Alert Company is a closely held investment-services group that has been very successful over the past five years, consistently providing most members of the top management group with 50% bonuses....
-
The Wilkinson catalyst chlorotris (triphenylphosphine) rhodium(I), ClRh(PPh0)3, brings about the catalytic hydrogenation of an alkene in homogeneous solution: (a) Using the following mechanistic...
-
Characterize each step of the mechanism in Eq. 18.42b in terms of the fundamental processes discussed in the previous section. Give the electron count and the oxidation state of the metal in each...
-
Characterize each step of the mechanism in Eq. 18.42b in terms of the fundamental processes discussed in the previous section. Give the electron count and the oxidation state of the metal in each...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


