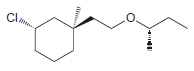
Identify the configuration of each chirality center in the following compounds: a. b. c. d. e. f.
Question:
a.
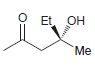
b.
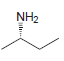
c.
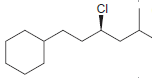
d.
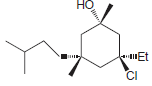
e.
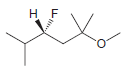
f.
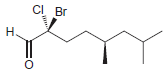
g.
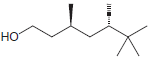
h.
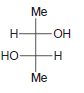
i.
Transcribed Image Text:
Et OH Me NH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
a b c ...View the full answer

Answered By

Antony Sang
I am a research and academic writer whose work is outstanding. I always have my customer's interests at heart. Time is an important factor in our day to day life so I am always time conscious. Plagiarism has never been my thing whatsoever. I give best Research Papers, Computer science and IT papers, Lab reports, Law, programming, Term papers, English and literature, History, Math, Accounting, Business Studies, Finance, Economics, Business Management, Chemistry, Biology, Physics, Anthropology, Sociology, Psychology, Nutrition, Creative Writing, Health Care, Nursing, and Articles.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Assign the configuration of each chirality center in the following compounds: (a) (b) (c) (d) (e) C=0 - - - H,OH %3D - - - H,OH
-
Seventeen of the 20 naturally occurring amino acids (See the following table) exhibit exactly one chirality center. Of the remaining three amino acids, glycine has no chirality center, and the other...
-
Each of the following compounds possesses carbon atoms that are chirality centers. Locate each of these chirality centers, and identify the configuration of each one: a. b. c. d. e. f. Ephedrine A...
-
A Contractors Ltd was formed on 1 January 2012 and the following purchases and sales of machinery were made during the first 3 years of operations. Each machine was estimated to last 10 years and to...
-
What recommendations would you make to Howard Schultz to sustain the company's growth and support continued strong financial performance in the years ahead?
-
Plot the given polar coordinate points on polar coordinate paper. (0.5,8.4)
-
What are the main features of cost-plus contract?
-
American Investor Group is opening an office in Portland, Oregon. Fixed monthly costs are office rent ($8,000), depreciation on office furniture ($1,800), utilities ($2,200), special telephone lines...
-
Question 1: Multiple Choice (5 marks) (A1) 1. Accounting generally has the responsibility for a. Setting company goals. b. Expressing the budget in financial terms. c. Enforcing the budget. d....
-
More than 50 million guests stayed at bed and breakfasts (B&Bs) last year. The Web site for the Bed and Breakfast Inns of North America (www.bestinns.com), which averages approximately seven visitors...
-
For each pair of compounds, identify which compound is more acidic and explain your choice. (a) 2,4-Dimethyl-3,5-heptanedione or 4,4-Dimethyl-3,5-heptanedione (b) 1,2-Cyclopentanedione or...
-
In Chapter 10, we will see that an acetylide ion (formed by treatment of acetylene with a strong base) can serve as a nucleophile in an S N 2 reaction: This reaction provides a useful method for...
-
Why doesnt gamma emission change the elemental identity of a nucleus?
-
You are the cost accountant of an engineering concern which has three departments - preparation, machining and assembly. The budgeted direct labour hours for the workshops are 8,000, 12,000 and...
-
What alternative to fostering fun and enjoyment at work do you think might have worked for Zappos?
-
Using the techniques of dimensional analysis, and assuming that experimentation shows the dimensionless number to be 1, derive the following equation: E v = Job card two The results of an ultrasonic...
-
Given the historical cost of product Carla Vista is $13, the selling price of product Carla Vista is $15, costs to sell product Carla Vista are $3, the replacement cost for product Carla Vista is...
-
What causes of outliers in statistics and when I create a boxplot why do I not see the outliers. What steps are to take in creating a boxplot?
-
In Problems 3946, find the midpoint of the line segment joining the points P 1 and P 2 . P = (2,-3); P = (4,2)
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
Predict the major product that is obtained when the following alkyl halides is treated with potassium terf-butoxide. Explain your reasoning. OCH CH CHI
-
Use a hybridization argument to predict the geometry of The neutral compound Pd(PPh3)4.
-
The Wilkinson catalyst chlorotris (triphenylphosphine) rhodium(I), ClRh(PPh0)3, brings about the catalytic hydrogenation of an alkene in homogeneous solution: (a) Using the following mechanistic...
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid
-
uppose a taxpayer has exhausted his lifetime exclusion amount and has $14 million. a. Assuming a flat 40% gift tax rate, what is the maximum amount a taxpayer can transfer to her daughter (and still...
-
Physical Units Method, Relative Sales Value Method Farleigh Petroleum, Inc., is a small company that acquires high - grade crude oil from low - volume production wells owned by individuals and small...

Study smarter with the SolutionInn App


