In each case below, draw a Newman projection as viewed from the angle indicated: (a) (b) (c)
Question:
(a)
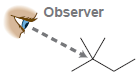
(b)
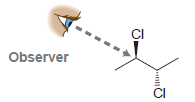
(c)
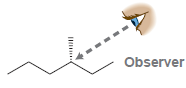
(d)

(e)
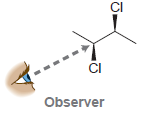
(f)
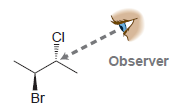
Transcribed Image Text:
Observer CI Observer CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
a b c ...View the full answer

Answered By

Anoop V
I have five years of experience in teaching and I have National Eligibility in teaching (UGC-NET) .
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider 2-methylpropane (isobutene). Sighting along the C2-C1 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. (c)...
-
Draw a Newman projection along the C2-C3 bond of the following conformation of 2, 3-dimethylbutane, and calculate total strainenergy:
-
Consider 2-methylbutane (isopentane) Sighting along the C2C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. (c)...
-
Verify each identity by comparing the graph of the left side with the graph of the right side on a calculator. sin 4x(cos 2x - sin2x) sin 8x 2
-
Given the scenario, your role and the information provided by the key players involved, it is time for you to make a decision. If you are finished reviewing this scenario, close this window and...
-
What types of training would Lynda.corn have difficulty providing customers? What kinds of education are less appropriate for e-leaming than traditional in-person courses? Lynda Weinman, cofounder of...
-
3. A university that follows the AICPA college model for internal control may have all of the following funds, except: a Property, plant, and equipment b Loan c Life income d Endowment
-
AAA Appliances Inc. has two production departments. The nature of the process is such that no units remain in process in Finishing at the end of the period. At the beginning of the period, 10,000...
-
You have been asked to prepare a December cash budget for Ashton Company, a distributor of exercise equipment. The following information is available about the company's operations: a. The cash...
-
There are 2 shinobis with chakra levels 5 and 10 respectively and the desired sum of chakra levels is utmost 15 Starting with ke0, suy of chakra levels after attack max(5-0,0) + max(10- 0,0) 5+10 15....
-
The following table indicates the number of constitutional isomers with molecular formula C 7 H 16 . Draw each of the isomers, making sure not to draw the same compound twice. NUMBER OF...
-
Draw a bond-line structure for each of the following compounds: (a) (b) (c) CH CH- CH2CH3 CH CH-CH CH . CH
-
The first 50 unit order of a job shop costs \(\$ 1,500\). It is believed that the shop experiences a \(75 \%\) learning curve rate. Determine a reasonable price quote for the next 80 unit order of...
-
The adjusted trial balance columns of a worksheet for Levitt Corporation are shown below. The worksheet is prepared for the year ended December 31, Complete the worksheet by (a) entering the adjusted...
-
Derive the commutator $\left[Q_{i}, Q_{j} ight]=i \epsilon_{i j k} Q_{k}$ for the charge defined in Eq. (33.4). Use the charge (33.4) to write the commutator, displaying explicit matrix indices...
-
Verify that the potential $V(\pi, \sigma)$ can be written as Eq. (33.11), and that if $\epsilon=0$ and the symmetry is implemented in the Wigner mode the masses for the $\pi$ and $\sigma$ fields are...
-
Figure 5.7 shows a number of yield curves at various points in time. Go to www.treasury.gov, and in the Resource Center at the top of the page click on Data and Charts Center. Find the Treasury yield...
-
The number of vacation days used by a sample of 20 employees in a recent year In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 3 9 2 17 5 3 2 2 6 4 0 10...
-
What is the horizontal line test and what does it indicate?
-
A copper rod of length L =18.0 in is to be twisted by torques T (see figure) until the angle of rotation between the ends of the rod is 3.08. (a) If the allowable shear strain in the copper is 0.0006...
-
Write structural formulas for each of the following: (a) 3-Nitrobenzoic acid (b) p-Bromotoluene (c) o-Dibromobenzene (d) m-Dinitrobenzene (e) 3,5-Dinitrophenol (f) p-Nitrobenzoic acid (g)...
-
Write structural formulas and give acceptable names for all representatives of the following: (a) Tribromobenzenes (b) Dichlorophenols (c) Nitroanilines (d) Methylbenzenesulfonic acids (e) Isomers of...
-
Which of the following molecules would you expect to be aromatic? (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) N+
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...
-
In the context of portfolio theory, what is diversification primarily intended to do ? A ) Increase returns. B ) Reduce risk. C ) Maximize tax efficiency. D ) Simplify investment management.

Study smarter with the SolutionInn App


