In each of the following pairs of compounds identify the compound that liberates the most heat upon
Question:
(a)
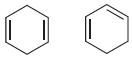
(b)
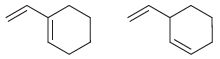
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
In each case ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following pairs of compounds undergoes a Bronsted acidbase reaction for which the equilibrium lies to the right. Give the products of each reaction, and identify the acid, the base, the...
-
Each of the following pairs of compounds undergoes a Bronsted acid-base reaction for which the equilibrium lies to the right. Give the products of each reaction, and identify the acid, the base, the...
-
Indicate whether each of the following pairs of compounds are identical or are enantiomers, diastereomers, or constitutional isomers: a. b. c. d. e. f. g. h. i. j. k. l. m. n. o. p. H C) and H H H CI...
-
Types of audit procedures Audit procedures are used to gatherevidence to support the auditors conclusions on the fairpresentation of a companys financial statements. Procedures can beperformed...
-
A saturated sample of clay with an SL of 20 has a natural water content of 32%. What would its dry volume be as a percentage of its original volume if s is 2.67?.?
-
In Exercises find the Maclaurin series for the function. f(x) = x sin x
-
Explain what information is conveyed by the following balances standing in the ledger of a restaurant at 31st December 2005 after balancing the accounts and preparing the final accounts for the year...
-
Mark Lawrencethe man with two first nameshas been pursuing a vision for more than two years. This pursuit began when he became frustrated in his role as director of human resources at Cutting Edge, a...
-
Exercise 5A-1 (Algo) High-Low Method (LO5-10) The Cheyenne Hotel in Big Sky, Montana, has accumulated records of the total electrical costs of the hotel and the number of occupancy-days over the last...
-
The need for a universally accepted theory of accounting Team 1: Argue that a universally accepted theory of accounting is needed. Team 2: Argue that a universally accepted theory of accounting is...
-
Based on what youve read in this chapter and the information on environmental economics in Chapter 2, explain how a global market for CO 2 permits would function.
-
Insurance companies that provide policies for hurricanes and other natural disasters may shift hundreds of millions of dollars of their investments from fossil fuels to solar energy. On the basis of...
-
What is a database?
-
The Intel Outsourcing case case explores the make-versus-buy decision for the well-known chip maker. Use The Strategic Sourcing framework to examine this important decision for Intel. Use the...
-
Final-year students enrolled in the Interactive Multimedia course at Edith Cowan University are required to develop skills and expertise in managing the design and development of client websites. The...
-
Find the minimum tractive effort required for vehicle to maintain 70mph speed at 5%upgrade through an air density of 0.002045 slug/ft^3. Show all steps and unit conversion please Problem 2:...
-
Sanburn writes about the conflict of decreasing funding and enrollment for community colleges and the increasing value of an associate degree. Explain how those two factors can co - exist at the same...
-
Rare beauty new Shampoo and Conditioner Branding Strategy What is the branding strategy for your organization? What is the purpose of your brand? How will you differentiate yourself from domestic...
-
Find each product. (y + 2)
-
Annual dividends of ATTA Corp grew from $0.96 in 2005 to $1.76 in 2017. What was the annual growth rate?
-
A 1.50 g sample of coniine, the toxic extract of poison hemlock, was dissolved in 10.0mL of ethanol and placed in a sample cell with a 5.00 cm path length. The observed rotation at the sodium D line...
-
Assign priorities to the following sets of sub stituents: (a) H, OH, CH 2 CH 3 , CH 2 CH 2 OH (b) CO 2 H, CO 2 CH 3 , CH 2 OH, OH (c) CN, CH 2 NH 2 , CH 2 NHCH 3 , NH 2 (d) SH, CH 2 SCH...
-
Orient each of the following drawings so that the lowest-priority group is toward the rear, and then assign R or Sconfiguration: (b) (a) (c) 2 2
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


