In the next chapter, we will learn a method for preparing alkynes (compounds containing C ¡ C
Question:
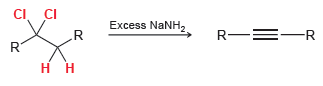
Transcribed Image Text:
CI. CI .R Excess NaNH, R-=-R R. R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
CI CI...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
We?ll see in the next chapter that alkynes undergo many of the same reactions that alkenes do. What product might you expect from each of the following reactions? 1 equiv Br2 CH3 (a) 2 equiv H2, Pd/C...
-
Consider the structure of anisole (also called methoxybenzene). In the next chapter, we will discuss whether a methoxy group is electron donating or electron withdrawing. We will see that there is a...
-
A capacitor being charged has a current carrying charge to and away from the plates. In the next chapter we will define current to be dQ/dt, the rate of charge flow. What is the current to a 10 F...
-
Boeing Company was scheduled to deliver several of its 747-400 jumbo jetliners to Northwest Airlines by December 31, 1988. Northwest set that deadline because it needed the $16 million in investment...
-
Critical Thinking: A number of currency crises have affected certain countries, which have also resulted in contagion in the sense that the crises affected neighboring countries. In a critical essay,...
-
In Exercises find a polar equation for the conic with its focus at the pole. (For convenience, the equation for the directrix is given in rectangular form.) Conic Hyperbola Vertex or Vertices ...
-
Explain how social media can produce sales revenues for a brand and compare the performance measures linked to costs versus revenues.
-
As you've seen in this chapter, activity based costing (ABC) systems are useful in helping companies make better decisions about pricing, product mix, and cost management related to product design...
-
The following information were taken from Bach Co.: Bach established a petty cash fund on May 2, 2021, amounting to P5,000. Expenditures from the fund by the custodian as of May 31, 2021, were...
-
Compute the correlation sequences rxx (l) and rxy (l) for the following signal sequences. 1. nu - N
-
Draw the mechanism for each of the following reactions: a. b. c. NaOMe CI NaOEt. Br
-
Identify whether each of the following reagents would be a strong nucleophile or a weak nucleophile, and also indicate whether it would be a strong base or a weak base: a.
-
Use the result of Exercise 73 and your knowledge of alternating series to show that Data From Exercise 73 When a voltage V is applied to a series circuit consisting of a resistor R and an inductor L,...
-
Context This task requires analysing a network scenario, design the network architecture and recommend IT solutions including ethical, security and sustainability considerations.The purpose of this...
-
What was the Prime Cost Percent for Mandy's BBQ Pit for August? Select one: a. 46.5% b. 73.9% c. 63.4% d. 85%
-
Finding Critical Values and Confidence Intervals. In Exercises 5-8, use the given information to find the number of degrees of freedom, the critical values x? and x*, and the confidence interval...
-
An investor sold 100 shares of ABC stock short at $25 and buys one ABC Jan 30 call @ $5. What is this investor's maximum gain, maximum loss, and breakeven points from this strategy?
-
Jake, Sachs and Brianne own a tour company called Adventure Sports. The partners share profits and losses in a 1:3:4 ratio. After Lengthy Dissagreements among the partners and several unprofitable...
-
Factor out the greatest common factor. (OI + 2) (S-2) + (L + ) (S-2)
-
What steps must a business take to implement a program of social responsibility?
-
Alkyl benzenes such as toluene (methylbenzene) react with NBS to give products in which bromine substitution has occurred at the position next to the aromatic ring (the benzylic position). Explain,...
-
Draw resonance structures for the benzyl radical, C6H5CH12, the intermediate produced in the NBS bromination reaction of toluene.
-
What product would you expect from the reaction of l-phenyl-2-hutene with NBS?Explain. 1-Phenyl-2-butene
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


