Alkyl benzenes such as toluene (methylbenzene) react with NBS to give products in which bromine substitution has
Question:
Alkyl benzenes such as toluene (methylbenzene) react with NBS to give products in which bromine substitution has occurred at the position next to the aromatic ring (the benzylic position). Explain, based on the bond dissociation energies in Table 5.3.
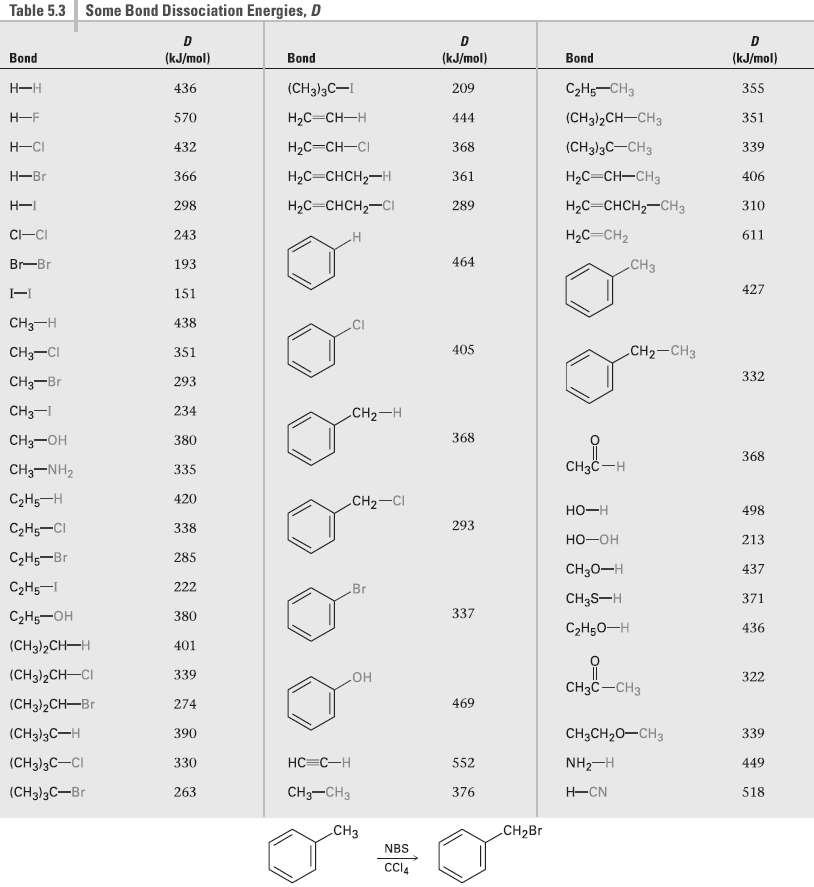
Transcribed Image Text:
Table 5.3 Some Bond Dissociation Energies, D (kJ/mol) (kJ/mol) (kJ/mol) Bond Bond Bond С-На —снз (CH3)3C-I Н—н 436 209 355 (CHg]2CH -CHз Н-F НаС —Сн—н 570 444 351 НаС— Сн—CI Н-СI (CH3)3C-CH3 432 368 339 Hас — снсH2—н Hас — Сн—сна Н—Br 366 361 406 Нас снсн—снз Hас - снсH2—сI Н- 298 289 310 Hас Сн2 C-CI 243 611 464 CHз Br-Br 193 427 151 CH3-H 438 CI CH— CHз 405 CH3-CI 351 332 CHз—Br 293 CHз-1 234 CH2-H CHз—он 368 380 368 Cнзс —н CHз-NH2 335 СаНs —Н 420 CH2-CI 498 но-н 293 СНо—СI 338 но-он 213 СаН — Br 285 CH30-H 437 СаНs — 222 Вг CH3S-H 371 337 СэН—он 380 СэHоо—н 436 (CH3)2Cн—н 401 (CH3)2CH-CI 339 322 CнзC —CHз но 469 (CH3)2CH-Br 274 (CH])3C—н 390 CH;CH20-CH3 339 НC -С —Н (CHд)3C—CI 330 NH2-H 552 449 CH—CHз H-CN (CH3)3C-Br 263 376 518 .CHз CH2B NBS CCI4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 35% (14 reviews)
Table 53 shows that the bond dissociation energy of C 6 H 4 CH 2 H is 368 kJmol This value is compar...View the full answer

Answered By

Marcus Solomon
I am committed to ensuring that my services always meet the clients' expectations.
4.60+
82+ Reviews
117+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF - (CH3)2CHF + H2O (b)...
-
Find examples of C-H bond dissociation energies in Table 10.1 that are as closely related as possible to the bonds to Ha, Hb, and Hc in the molecule at right. Use these values to answer the questions...
-
Which alkyl halide would you expect to react more rapidly by an SN2 mechanism? Explain your answer. (a) (b) (c) (d) (e) Br r or or CI
-
Write MATLAB code of the question. Do all parts and show code with comments and also attach a screenshot of code and outputs. Using the results of Problem 4.13, verify the following properties of WN...
-
a. Consider a recommendation that the prices for the Educational, Large-Scale, and High-Speed versions should be $75, $275, and $475, respectively. What annual profit would these prices achieve? b....
-
A gas separation process has been proposed to remove selectively two pollutants, hydrogen sulfide (H 2 S) and sulfur dioxide (SO 2 ), from an exhaust gas stream containing 3.0 mole% H 2 S, 5.0 mole%...
-
On January 1, 2004, Yorkville Hotels Corporation had Retained Earnings of $580,000. During the year, Yorkville had the following selected transactions. LO5 1. Declared cash dividends $120,000. 2....
-
Alfred Engineering Company is a young and growing producer of electronic measuring instruments and technical equipment. You have been retained by Alfred to advise it in the preparation of a statement...
-
Periodic Inventory by Three Methods: Cost of Merchandise Sold The units of an item available for sale during the year were as follows: Jan. 1 Inventory Purchase 30 units 5108 70 units $120 Mar. 10...
-
Eckholm Company records all prepayments in income statement accounts. At April 30, the trial balance shows Supplies Expense $2,800, Service Revenue $9,200, and zero balances in related balance sheet...
-
What product(s) would you expect from the reaction of 1, 4-hexadiene with NBS? What is the structure of the most stable radical intermediate?
-
Draw resonance structures for the benzyl radical, C6H5CH12, the intermediate produced in the NBS bromination reaction of toluene.
-
In Exercises solve for x accurate to three decimal places. elnx = 4
-
MAT 152 Project 3: MLB Team Salaries The data set below is the total salary of each Major League Baseball (MLB) team salaries per team in 2016. Find the probabilities for normal distributions and...
-
deficit, surplusincreased, decreased$795, $1,975, $54,635, $35 6. Cash-flow statement Sam and Joan Wallingford have been married for two years. They have been trying to save but feel that there is...
-
Trudy bought the vacant lot adjacent to her house and planted a large garden there. The garden produces more vegetables than her family needs, and Trudy earns some extra cash by selling them at a...
-
Requirements Medical researchers once conducted experiments to determine whether Lisinopril is a drug that is effective in lowering systolic blood pressure of patients. Patients in a treatment group...
-
1. Balroop while looking for Gurjap walks 315m [N] toward the forest, then 133 m [28 S of E] through it, and finally finds him deep inside the forest after walking another 55 m [ 31 S of W]....
-
How might Alma have better prepared her loyal team for taking over orientation and training responsibilities? Which aspects of orientation and training should a restaurants owner or manager be...
-
6 (a) Briefly develop a mathematical model of the behaviour of a copper-twisted pair cable (b) Derive the magnetic energy from: w given that: K + w, where the - - k symbols have their usual meaning...
-
Consider the generic ionic compounds with the formulas AX and BY and the following solubility rules: AX soluble; BY soluble; AY soluble; BX insoluble Let circles represent A+ ions; squares represent...
-
In the presence of a small amount of bromine, the following light-promoted reaction has been observed. (a) Write a mechanism for this reaction. Your mechanism should explain how both products are...
-
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed. (a) Cyclo-hexane (b)...
-
When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of...
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...
-
Exercise 1 1 - 7 ( Algo ) Net present value and unequal cash flows LO P 3 Gomez is considering a $ 2 1 0 , 0 0 0 investment with the following net cash flows. Gomez requires a 1 2 % return on its...
-
a Campbell Inc. produces and sells outdoor equipment. On July 1, 2011. Campbell issued $40,000,000 a 10-year, 10% bonds at a market (effective) interest rate of 9%, receiving Cash of 548,601,480....

Study smarter with the SolutionInn App


